USPSTF recommends that menopausal hormone therapy not be used to prevent chronic conditions
The U.S. Preventive Services Task Force (USPSTF) makes recommendations on clinical preventive services to primary care clinicians. • The USPSTF scope for clinical preventive services includes: * screening tests * counseling * preventive medications • Task force services are offered in a primary care setting • Recommendations apply to adults and children with no signs or symptoms • The task force makes recommendations based on rigorous review of existing peer-reviewed evidence • The task force does not conduct the research studies, but reviews and assesses the research • The USPSTF evaluates benefits and harms of each service based on factors such as age and sex • The task force is an independent panel of nonfederal experts in prevention and evidence-based medicine
Based on review of 51 articles published since 2002, the USPSTF recommends against using combined estrogen-progestin or estrogen-only hormone therapy (HT) to prevent chronic conditions such as coronary heart disease or osteoporosis ("D" recommendation).(1) See grade definitions below.(2) The USPSTF emphasizes that these recommendations do not apply to treatment of menopausal symptoms such as hot flashes or vaginal dryness, and do not apply to menopausal women younger than 50.(3)
The current report acknowledges that, although HT prevents fractures in menopausal women, the excess risks for stroke, venous thromboembolism (VTE), breast cancer (in women taking combination estrogen-progestin HT), dementia, gallbladder disease, and urinary incontinence outweigh this benefit.
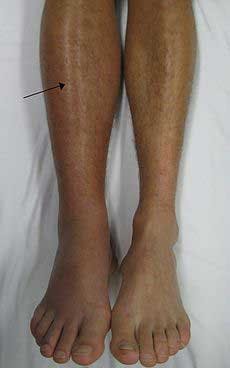
A deep vein thrombosis in the right leg. Note the swelling and redness.
A comment by Andrew M. Kaunitz, MD, stated his concern that these new USPSTF recommendations will discourage selective use of hormone therapy to prevent osteoporosis. HT's efficacy in preventing bone-mass loss, osteoporosis, and osteoporotic fractures is documented by randomized trials and observational data. The use of HT to prevent osteoporosis has been approved by the FDA and is consistent with the North American Menopause Society's 2012 Position Statement. Dr. Kaunitz cites an article by Diane E. Judge, APN/CNP, in Journal Watch.(4) Dr. Kaunitz also cites an article about ultralow doses of estrogen prevent loss of bone mass in menopausal women.(5) He states that, in his practice, some women who have been using HT for symptom relief and who have "outgrown" their symptoms choose to continue HT at a lower dose to take advantage of its benefits to bones.(6) Although excess risk for venous thromboembolism clearly accompanies use of oral HT, observational studies have consistently shown that transdermal estrogen regimens do not raise VTE risk. He cites a retrospective study that shows that risk for venous thromboembolism is lower with transdermal estrogen than with oral estrogen.(7,8,9). He concludes that, low- or ultralow-dose HT, transdermal when feasible, is a reasonable option for women at above-average risk for osteoporosis, such as smokers and white or Asian women who are slender. As well, in contrast to this USPSTF recommendation, a 2012 Cochrane review supports use of HT to prevent osteoporosis in selected women.(10) One caveat about HT for preventing osteoporosis is that the protection is relatively short-acting, unlike that of bisphosphonates. Accordingly, high-risk women who discontinue HT should receive ongoing attention to skeletal health. “HT is not indicated for primary or secondary prevention of cardiovascular disease or dementia, nor for preventing deterioration of cognitive function in postmenopausal women. Although HT is considered effective for the prevention of postmenopausal osteoporosis, it is generally recommended as an option only for women at significant risk, for whom non‐oestrogen therapies are unsuitable. There are insufficient data to assess the risk of long term HT use in perimenopausal women or postmenopausal women younger than 50 years of age”.(10) Notwithstanding this guidance, HT's role in averting osteoporosis should not be overlooked.References 1. Moyer VA on behalf of the U.S. Preventive Services Task Force. Menopausal hormone therapy for the primary prevention of chronic conditions: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med 2012 Oct 23; [e-pub ahead of print]. (http://annals.org/article.aspx?articleid=1384872) 2. U.S. Preventive Services Task Force, Grade Definitions, Strength of Recommendations. http://www.uspreventiveservicestaskforce.org/3rduspstf/ratings.htm. 3. The North American Menopause Society. The 2012 hormone therapy position statement of the North American Menopause Society. Menopause 2012 Mar; 19:257. 4. http://womens-health.jwatch.org/cgi/content/full/2012/329/1. 5. Ettinger B, Ensrud K, Wallace R, Johnson K, Cummings S, Yankov V, Vittinghoff E, and Grady D. Effects of Ultralow-Dose Transdermal Estradiol on Bone Mineral Density: A Randomized Clinical Trial. Obstetrics & Gynecology. September 2004 - Volume 104 - Issue 3 - pp 443-451. http://journals.lww.com/greenjournal/Fulltext/2004/09000/Effects_of_Ultralow_Dose_Transdermal_Estradiol_on.4.aspx. 6. Published in Journal Watch Women's Health October 25, 2012. http://womens-health.jwatch.org/. 7. Further Evidence That Route of Estrogen Administration Affects VTE Risk. JW Womens Health Dec 1 2011. http://womens-health.jwatch.org/cgi/content/full/2011/1201/2. 8. Laliberté F et al. Does the route of administration for estrogen hormone therapy impact the risk of venous thromboembolism? Estradiol transdermal system versus oral estrogen-only hormone therapy. Menopause 2011 Oct; 18:1052. 9. Canonico M and Scarabin P-Y. Further evidence for promoting transdermal estrogens in the management of menopausal symptoms. Menopause 2011 Oct; 18:1038. 10. Marjoribanks J, Farquhar C, Roberts H, Lethaby A. Long term hormone therapy for perimenopausal and postmenopausal women. Cochrane Database of Systematic Reviews 2012, Issue 7. Art. No.:CD004143. DOI: 10.1002/14651858.CD004143.pub4.