The new guidelines for prophylactic antibiotic use are finally here!
This article will review the highlights of the report, “The use of prophylactic antibiotics prior to dental procedures in patients with prosthetic joints,” but you’re encouraged to read the entire ADA report.
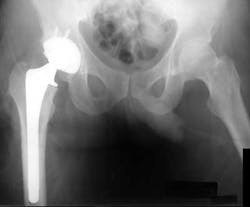
Arthroplasty is a surgical procedure to restore the integrity and function of a joint. Biofilms are an important consideration in the pathogenesis of prosthetic joint infections.(3) Organisms within biofilm become resistant to therapy, and as a result, antimicrobial therapy is often unsuccessful unless the biofilm is physically disrupted or removed by surgical debridement. This is similar to biofilm in the oral cavity, and we always perform debridement whether or not we use an antimicrobial.
In order to produce the guidelines, an extensive literature review was conducted. The expert panel used a rating system that balanced the level of certainty (low, moderate, high) and net benefit to achieve the strength of the clinical recommendation. The categories were: benefit outweighs potential harm; benefit balanced with potential harm; no benefit, potential harm outweighs potential benefit; or, no association. See Table 2 in the document for more information on the rating system.(4)
The 2014 panel approved clinical recommendations by means of a unanimous vote. The practical implications and conclusions resulted in the following clinical recommendation: “In general, for patients with prosthetic joint implants, prophylactic antibiotics are not recommended prior to dental procedures to prevent prosthetic joint infection. The practitioner and patient should consider possible clinical circumstances that may suggest the presence of a significant medical risk in providing dental care without antibiotic prophylaxis, as well as the known risks of frequent or widespread antibiotic use. As part of the evidence-based approach to care, this clinical recommendation should be integrated with the practitioner’s professional judgment and the patient’s needs and preferences.”(4)
A bit of history is in order. In 2012, a panel of experts representing the American Academy of Orthopaedic Surgeons (AAOS) and the American Dental Association (ADA) published a systematic review and associated clinical practice guideline entitled, “Prevention of Orthopaedic Implant Infection in Patients Undergoing Dental Procedures: Evidence-based Guideline and Evidence Report.”(6) While all recommendations are intended to be part of clinical decisions based on consultation of the practitioner and patient, the recommendations in 2012 were, “The practitioner might consider discontinuing the practice of routinely prescribing prophylactic antibiotics for patients with hip and knee prosthetic joint implants undergoing dental procedures.” The recommendation was inconclusive and caused much confusion in the medical and dental communities. “We are unable to recommend for or against the use of topical oral antimicrobials in patients with prosthetic joint implants or other orthopaedic implants undergoing dental procedures.”(6) Hence, the formation of the new guideline. (Note: Italics were placed by the author of this article.)
There are those who feel they should “err on the side of caution” and still recommend antibiotics to patients with prosthetic joints. Keep in mind that antibiotic resistance, adverse drug reactions, and cost are concerns that bolster the argument against antibiotic prophylaxis. Antibiotics can possibly cause death in susceptible patients. An example is that the antibiotic penicillin is the most recurrent cause of anaphylaxis (due to pharmaceuticals) in humans, and the use of penicillin is the cause of almost 75% of fatal anaphylaxis cases in the United States each year.(4,7)
Systemically developed clinical practice guidelines (CPGs) are intended to structure current medical knowledge in a way that will support health-care providers in delivering high quality care. Although a professional society may have a disclaimer that guidelines are not intended or created to set legal precedent, CPGs are being used in the malpractice area to delineate a reliable standard of care.(8) It is sometimes claimed that CPGs may be used with greater effect for the plaintiff's guilt rather than by the defense as a means to exonerate the defendant. Clinicians should be aware of the legal use of CPGs and the related risk management implications. If unsure of the correct action to take, you may want to consult an attorney.
This new guideline, an evidence-based clinical practice guideline, was compiled by a panel of experts and is the result of a comprehensive systematic review, and provides oral health care and medical professionals with much needed guidelines in the area of prophylactic antibiotics prior to dental procedures in patients with prosthetic joints. The new guideline article has an accompanying online continuing education activity available on the website.(9)
References
1. Kurtz S, Ong K, Lau E, et al. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am 2007; 89:780.
2. Widmer AF. New developments in diagnosis and treatment of infection in orthopedic implants. Clin Infect Dis 2001; 33 Suppl 2:S94.
3. Berbari EF, Hanssen AD, Duffy MC, et al. Risk factors for prosthetic joint infection: case-control study. Clin Infect Dis 1998; 27:1247.
4. Sollecito TP, Abt E, Lockhart PB, et al. The use of prophylactic antibiotics prior to dental procedures in patients with prosthetic joints. The Journal of the American Dental Association, Volume 146, Issue 1, Pages 11-16.e8 (January 2015). DOI: 10.1016/j.adaj.2014.11.012.
5. Broughton R, Rathbone B. What makes a good clinical guideline? http://www.medicine.ox.ac.uk/bandolier/painres/download/whatis/whatareclinguide.pdf.
6. American Academy of Orthopaedic Surgeons and American Dental Association. Prevention of orthopaedic implant infection in patients undergoing dental procedures: evidence-based guideline and evidence report.American Academy of Orthopaedic Surgeons, Rosemont, IL; 2012. www.aaos.org/research/guidelines/PUDP/PUDP_guideline.pdf.
7. Neugut AI, Ghatak AT, Miller RL. Anaphylaxis in the United States: an investigation into its epidemiology. Arch Intern Med. 2001; 161: 15–21.
8. Moses RE, Feld AD. Legal risks of clinical practice guidelines. Am J Gastroenterol. 2008 Jan;103(1):7-11. doi: 10.1111/j.1572-0241.2007.01399.x.
9. http://jada.ada.org/ce/home.