Does pH Matter? The role of water in dental patients' diets
Abstract
This paper explored research that discussed the differences between an alkaline versus an acidic diet and its effects on overall health. In addition, the numerous health benefits of the alkaline diet were explored, such as healthy bones, higher osteoblast activity, bone remineralization, preservation of muscle mass, reduction of tumor invasiveness and metastasis, and improvement in the excretion of toxins.
The research also examined the potential hydrogen (pH) of popular bottled waters and the health promotion factors of alkaline water to the body. The influence of the reduction of the bacterial load in the digestive tract through good oral hygiene, flossing of teeth, properly chewed food, and bowel evacuation were also considered. Alkaline water, tap water, and acidic water were examined based on oral and overall health.
Different reasons were explained as to why bottled water sales have spiked. The reason behind bottled water preference between males versus females was investigated. In acid reflux, alkaline water was used as a therapeutic agent to prevent more harmful side effects. Finally, the Basic Erosive Wear Examination (BEWE) index was explained in relation to oral hygiene and an acidic diet.
Keywords: alkaline diet, acidosis, water, bottled water, potential Hydrogen (pH), Basic Erosive Wear Examination (BEWE), potential renal acid load (PRAL), acid reflux, osteoporosis, oral hygiene
Does pH matter?
There is a fluctuation of pH that is constantly being altered in the body in order to maintain homeostasis. The pH scale ranges from zero to fourteen. A pH of seven is considered neutral, and a number below seven is acidic and a number above seven is alkaline. A food is considered acidic or alkaline as determined by the potential renal acid loads (PRALs). PRAL is defined by Collison (2010) as a calculated value of certain nutrients in food that have the most significant indication of changing the acidity or alkalinity of the body (Collison, 2010).
It has been shown that the diet whether alkaline or acidic has different effects on the body. Water has varying degrees of pH and benefits, depending on the source. BEWE, or basic erosive wear examination for dental care professionals can have a positive influence on patient care and can help promote a state of well-being.
Alkaline diet versus an acidic diet
Research has shown that the body’s pH has the potential to be altered by the diet; this is dependent on the type of foods eaten. Some foods leave acidic or alkaline by-products in the body (Mousa, 2016). Fried foods, eggs, dairy, meat, fish, processed foods, alcohol, soda, most grains, some legumes such as beans and peas, and chocolate are included as acid-forming foods. Fruits, vegetables, legumes such as green beans, seeds, spices, and certain grains such as quinoa and wild rice are included as alkaline-forming foods.
According to Schwalfenberg (2012), a food’s pH can be affected by the soil that they are grown in by changing the mineral content. Minerals found in foods are used as buffers to maintain pH. The ideal pH of the soil that plants are grown in is between 6 and 7; this allows for the best overall availability of essential nutrients. “Acidic soils below pH of 6 can have reduced amounts of calcium and magnesium, and soil above a pH of 7 can result in chemically unavailable iron, manganese, copper, and zinc” (Schwalfenberg, 2012).
An alkaline diet, a popular fad, was “based on the fact that certain foods can affect the acidity of body fluids, including the urine or blood and can, therefore, be used to treat or prevent diseases” (Mousa, 2016). An acidic diet coincided with the typical American diet which was rich in saturated fats, sodium, simple sugars, and chlorides.
Systemic effects
There is constant work that is performed by the body to maintain homeostasis. The pH range in the extracellular fluid would be about 7.35 to 7.45, and it is maintained through the body’s respiratory and renal system. The excreted product of carbon dioxide by the respiratory system, and the excreted product of a noncarbonic acid or base by the renal system (Mousa, 2016).
According to Mousa (2016), “Everyday metabolism produces acid as nonvolatile sulfate from amino-acid catabolism, nonmetabolized organic acids, and phosphoric and other acids. The kidney reabsorbs all of the filtered bicarbonate and generates new bicarbonate in the collecting duct.” In a normal conditioned state, the quantity of acid secreted and the renal generation of new bicarbonate equals the rate of metabolic proton generation, in turn preserves the pH balance.
Low level metabolic acidosis can be caused by a diet that is more acidic in nature. “Metabolic acidosis occurs when the body produces too much acid, and can also occur when the kidneys do not remove enough acid from the body” (Martin, 2015). The length of time that acidosis is present could be significant. Low-grade acidosis that is less severe, but chronic is thought to be brought about by two factors: advanced age and diet (Mousa, 2016).
In advanced age, there is a loss of renal acid-base regulatory function and a resultant increase in diet-induced metabolic acidosis while on the modern diet (Schwalfenberg, 2012). Acidosis can be promoted through the diet by its net acid load and by its sodium-chloride content (Mousa, 2016). The diet of today is considered poor in magnesium, potassium, and fiber while rich in saturated fat, simple sugars, sodium, and chloride. The ratio of potassium (K) to sodium (Na) in the diet has changed from previously 10 to 1 in the preagricultural period to now 1 to 3.
Sodium (Na+), potassium (K+), calcium (Ca2+), and magnesium (Mg2+) are considered cations that are found in alkaline food sources or supplements; they cause a decreased amount of calcium in the urine and an exerted protective effect on bone (Mousa, 2016). Phosphate (PO4-), sulfate (SO4-), chloride (Cl-), and organic acids are considered anions that are found in acidic foods or supplements that cause metabolic acidemia and increased calciuria when consumed in excess which was found to be harmful to bone health (Mousa, 2016). Sodium and Potassium both belong to the alkaline category; however, the movement toward a higher potassium intake ratio could have favorable health benefits (Mousa, 2016).
The production of sulfate and phosphate have shown to be detrimental to bone health and can be found in protein and grain foods; while fruits and vegetables are bone protective because of their potassium organic anion content (Mousa, 2016). A study performed by Barzel and Massey (1998) concluded, “Excessive dietary protein from foods with high potential renal acid load adversely affects bone, unless buffered by the consumption of alkali-rich foods or supplements.”
Another study suggested that acidosis stimulates resorption of bone by activating mature osteoclasts, instead of forming new osteoclasts; this further supports how critical an acid-base balance is in controlling osteoclast function (Meghji, 2001).
A diet high in acid-forming products forces the body to borrow minerals from vital organs and bones to neutralize the acid, so it can be safely removed from the body (Collison, 2005). This can cause the body to have severe and prolonged damage because of the high acidity, which can go undetected for many years. Osteoporosis is a gradual process that takes place over decades, and remains undetected unless a bone density study is performed, or until a fracture occurs (Collison, 2005). Consuming a rich alkaline-forming diet has shown improvement in bone mineral density, muscle mass, reduced tumor-cell invasion and metastasis, protection from chronic illnesses, and optimal excretion of toxins from the body (Mousa, 2016).
There is an increase in the loss of muscle mass as we age. This loss of muscle mass can predispose a person to falls and fractures (Schwalfenberg, 2011). “In one three-year study, a diet rich in potassium, such as fruits and vegetables, as well as a reduced acid load, resulted in preservation of muscle mass in older men and women” (Schwalfenberg, 2011). An accelerated breakdown in skeletal muscle was noted in conditions such as chronic metabolic acidosis and chronic renal failure. By correcting acidosis, preservation of muscle mass may occur in conditions such as diabetic ketosis, trauma, sepsis, chronic obstructive lung disease, and renal failure where muscle wasting is common (Schwalfenberg, 2011).
Tumor-cell invasion and metastasis in vitro and in vivo has been shown to be stimulated by an acidic pH. While an increase in the pH of tumors selectively and the reduction of the formation of spontaneous metastases in mouse models of metastatic breast cancer has been shown through the use of oral sodium bicarbonate. An increase in extracellular pH significantly has been shown with the treatment regimen of oral sodium bicarbonate (Mousa, 2016).
When there is an increase in the dietary acid load, this can cause a disruption to the body’s acid-alkaline homeostasis. “This can result in chronic disease through the repeated borrowing of the body’s alkaline reserves” (Mousa, 2016). There can be more effective excretion of toxins from the body when there is an adjustment in the tissue’s alkalinity (Mousa, 2016).
An analysis in 2012, by Koufman and Johnston exposed that reflux disease is rising at about 4% since 1976. About 40% of the United States population has some form of reflux disease. It was proposed in the analysis that there was a positive correlation between reflux, cancer, and an acidic diet. It was seen that a pH of five in the larynx could cause damage in patients with laryngopharyngeal reflux. During digestion, pepsin broke down proteins into peptides. Pepsin was only activated at or below a pH of 6.5, but was optimized at a pH of two (Koufman & Johnston, 2012).
Research has shown that one in 20 patients with laryngopharyngeal reflux had tissue bound pepsin. When pepsin is tissue bound the important protective proteins were reduced dramatically. This then created an increase of a cellular component corresponding to laryngeal cancer. Antireflux therapy has shown to establish pharyngoesophageal tissue and aid in elimination of tissue bound pepsin. Human pepsin was studied by Koufman and Johnston to show the effectiveness of alkalizing water as therapeutic agent in reflux disease patients. The results immediately indicated that pepsin was denatured by natural artesian water with a pH of 8.8, unlike water tested with a neutral pH.
Denatured tissue bound pepsin was concluded to be beneficial for individuals to prevent damage to the laryngopharynx and could decrease the risk for cancer associated with reflux disease. The same natural artesian water was effective in buffer hydrochloric acid (stomach acid) that has a extremely low pH. In sufferers with acid reflux, using alkaline water along with an alkaline diet could be beneficial to decrease symptoms associated with acidity in the oral cavity and the esophagus (Koufman & Johnston, 2012).
Digestive Tract Bacterial Load
The most prominent places that have bacterial overgrowth have been found in the mouth, pharynx, and large intestine. It seems that oral and pharyngeal microorganisms have been shown to play a significant role in producing an acid load in the bloodstream; this is influenced by bad oral hygiene and unhealthy teeth and/or gums. This could be the link between bad oral hygiene and cardiovascular disease.
A study was done that included 104 patients of both genders between the ages of 50 and 90 years old. The study revealed a relationship between patients with hypertension and recent myocardial infarctions with the bad condition state of their oral cavities. A study has also revealed that people who have periodontal disease have an increased chance of developing cardiovascular disease (Golebiewska, Taraszkiewicz, and Kuklinska, 2006).
Improperly digested foods could lead to an overgrowth in bacteria that produce high acid. The production of high acid could happen if large food particles arrive in the large intestine without being properly chewed. A correlation has been shown between drinking large amounts of water during or immediately after a meal, and the dilution of stomach acids and enzymes. When additional food has been eaten after a main meal, there is a mixture of digested and undigested food which are then propelled toward the duodenum. High acids and toxins are produced when there is delayed bowel evacuation.
Acid production in the oral cavity can be reduced through the use of floss, wooden or plastic picks because food particles and plaques are removed that breed bacteria. “A cycle could happen in a sequential and cumulative pattern as follows-poor oral hygiene, gum disease, teeth plaques, teeth caries, teeth loss, and, finally, ineffective chewing” (Mousa, 2016). A study performed by Holmlund, Holm, and Lind (2006) showed a linear trend between the severity of periodontal disease and antihypertension treatment. Periodontal disease has also been shown to play a role in metabolic syndrome and pre-eclampsia (Holmlund, Holm, & Lind, 2006).
Probiotics was defined as microbial harmony between a host and live bacteria or yeasts cells. Foods containing probiotics are yogurts, kefir, and some other dairy products. Barbu et al. (2016) researched benefits of probiotics on oral health. In the mouth, cavities occurred because the oral cavity had an acidic bacterial environment. Studies suggested that lactobacillus, and bacillus found in probiotics can help cure or alleviate signs or symptoms of periodontal disease. Some different types of lactobacillus have anti-inflammatory properties, and can decrease harmful organisms (Barbu et al, 2016).
Water experiment
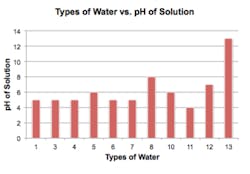
The authors of this paper conducted two experiments. The first was dismissed from the research because of possible bias and the drying time of litmus paper. Also, two of the variables tested were excluded in the second experiment due to availability. The second experiment is shown in Figure 1.
Figure 1:This figure illustrates the pH of nine different types of popular bottled water, city of McKinney, Texas, tap water, and an alkaline concentrate used in water.
The purpose of the experiment was to test the pH values of nine different popular brands of bottled water. Tap water and an alkaline spray were also included in the experiment. First, the unopened bottles of water stored at room temperature were labeled with numbers. Next, ten dispensing cups were lined up next to the unopened bottles of water, and were filled to the 15mL line with corresponding bottle of water or tap water. Gloved hands were used to prevent contamination of the dispensing cups and litmus paper. Next, the litmus paper was placed into the water and immediately taken out and placed on a piece of white paper.
After all nine bottles of water, tap water, and alkaline spray were tested, the litmus paper was left to air dry. The litmus paper was then compared next to the corresponding pH color chart to determine the pH of the solution. Bottle eleven had a pH of four. Bottles one, three, four, six, and seven had a pH of five. Bottles five and ten had a pH of six. Tap water had a pH of seven, bottle eight had a pH of eight, and the alkaline spray had a pH of 13.
Based on the results of the experiment, it was concluded that of the bottled waters tested, the most acidic water was bottle number eleven and the most alkaline was bottle eight. The alkaline spray had the most alkaline value, because the concentrate was sprayed directly on the litmus paper. The alkaline spray was then used to test the effect of the alkaline spray on the bottled water with the lowest pH. The alkaline spray was used based on the directions on the bottle, the pH was tested using the same litmus strips. The alkaline spray was sprayed directly into a cup containing water with the pH of four. The test strip was then placed into the solution and the strip was confirmed to have increased to a pH of seven.
Water
Bottled water has gained popularity in America in the last decade. In fact, Saylors, Prokopy, and Amberg (2010) explained that the United States was the number one consumer of bottled water in the world. Together, Americans have consumed between 8 to 9 billion gallons of bottled water a year. The cost for bottled water compared to tap water was steep because the price of a gallon of bottled water was 10,000 times that for tap water (Saylor, Prokopy, & Amberg, 2011).
Why are more people choosing bottled water? Is bottled water cleaner, safer, or healthier for consumers than tap water? Over the years, bottled water has been rumored to be safer to drink than tap water. Some researchers argued that marketing of bottled water may or may not have reduced public trust in tap water (Saylor, Prokopy, and Amberg, 2011). The truth was that tap water has to be regulated by the Environmental Protection Agency (EPA). The EPA “[set] rigorous rules for a wide variety of impurities and [acted] quickly when thresholds are passed” (Lewis, 2010). Meanwhile, the Food and Drug Administration (FDA) regulated bottled water, which did not have as strict regulations as the EPA.
Although the FDA did restrict some pollutants, such as lead, more stringently than the EPA, such regulations apply only to bottled water marketed across state lines (Lewis, 2010). In fact, about “60-70% of America’s bottled water was exempt from federal regulation” (Saylor, Prokopy, & Amberg, 2011).
It was argued that bottled water was not as safe as tap water due to more stringent laws pertaining to tap water, as well as faster reporting times required if any issues were to arise during the purification process. The decreased regulation on bottled water had lead to a rise in manufacturing bottled water (Saylor, Prokopy, and Amberg, 2011).
Saylor, Prokopy, and Amberg (2011) interviewed twelve males and ten females on Purdue campus about their drinking water sources. Seven out of the twelve males questioned chose unfiltered tap water as their primary drinking water source, while zero out of ten females chose unfiltered tap water as their primary drinking water source. Six out of ten females primary chose bottled water as their primary drinking source and one out of twelve males chose bottled water as their primary drinking source. Of these same interviewees 62% had testified drinking one or more bottles of water a week (Saylor, Prokopy, & Amberg, 2011).
A questionnaire was given to a different group of individuals in the same population to evaluate their stance regarding drinking water quality, drinking water safety, and their trust in their tap water source versus bottled water. Many individuals who chose bottled water over tap water responded that tap water was not as safe as bottled water, or bottled water tastes better than tap water. One interviewee responded that “I [saw] how much a bottle of water costs and I [thought] that they’d have to put a lot of time and effort into making sure it’s pure… [it was] just general knowledge that bottle water was just safer than tap water” (Saylor, Prokopy, & Amberg, 2011).
Tap water has been studied as an adjunct to improve skin composition. An experiment done by Williams et al. (2007), tested the effects of a mineral water with an acidic pH of 5.8 and tap water with a pH of seven on skin. The individuals studied in the experiment drank two liters of either of the waters for two weeks. One of the effects tested in the experiment tested skin density which increased significantly in those who drank tap water; while subjects who drank bottled mineral water had a significant decrease in their skin density (Williams et al., 2007).
Inorganic salts and trace amounts of organic matter present in solution in water were termed total dissolved solids (TDS). The components of TDS were found to be primarily cations such as calcium, magnesium, sodium, and potassium; the anion component consisted of carbonate, hydrogencarbonate, chloride, sulfate, and nitrate. It has become popular for people to consume reverse osmosis water, and the TDS range was found to be 80 to 110 with a pH range of 6.8 to 7.2 (Mousa, 2016).
It was found that reverse osmosis water was deficient in essential alkaline minerals when compared to water from natural springs and rivers. Bottled water has shown to be different from natural mineral water because of the specific geographical origin that the mineral water was obtained; it had original purity and the composition of minerals was stable. It had to be bottled at the source to prevent any chemical property alterations (Mousa, 2016).
The consumption of high mineral components in water has been seen to produce alkaline by-products in the blood. TDS concentrations from natural sources have been found to vary from fewer than 30 to 6000, which was dependent on different geographical regions. The World Health Organization has stated that the optimum pH of drinking water is in a range of 6.5 to 9.5.
It was studied that higher TDS concentrations in the drinking water solution had a lower incidence of coronary heart disease, arteriosclerotic heart disease, cardiovascular disease, and cancer; also, lower total mortality rates. A study was performed that consisted of 30 females dieticians aged 26.3 years, it revealed that alkaline mineral water significantly reduced bone resorption while calcium rich water had no effect on bone resorption (Mousa, 2016). It was concluded by Wynn et al. (2009) that the best waters for bone health are rich in both bicarbonate and calcium, and low in sulfate (Wynn et al., 2009).
In a study performed with mice that had been experimentally induced with diabetes showed improved blood glucose control when supplemented with alkaline ionized water. The alkaline ionized water seemed to provide an antioxidant defense mechanism in pancreatic beta cells (Kim & Kim, 2012). These results suggest that alkaline ionized water may help to function as an effective oral antidiabetic agent.
Dental hygiene considerations
As stated by Wright (2015), dental erosion was determined as diminished tooth structure caused by chemicals. The average mouth had a pH of 6.3 but when the oral cavity’s pH decreased below 5.5 demineralization of enamel begins, and the lower the pH of ingested substances the increased rate of demineralization. Wright also included that patients with braces, breath through their mouth, or have xerostomia resulting from decreased saliva (which can aid in buffering acids in the mouth) have shown to have a higher chance of erosion. Naturally occurring alkaline water that has a pH above seven can be beneficial in treating acid reflux and even slow progression of hypertension, muscle wasting and strokes (Wright, 2015).
An article published in the British Dental Journal (2013) contained a survey completed by dental professionals concluded that 83.5% have witnessed acidic wear in their office on a weekly basis. A rise in patients with acidic wear by about 86% was noticed in the same group of dental professionals (Acid wear headlining at oral health conference, 2013). According to Bartlett, Ganss, and Lussi (2008), oral health care providers may be the first ones to notice the effect of an increased acidic diet in patients. The maxillary palatal surface of teeth were the most commonly affected surface in those with reflux disease (Yuichi et al., 2015).
The BEWE has been an advantageous index used to assess dental erosion in the dental field and can be a great tool for clinicians. The basis behind the index was that the tooth with the sign of the most acidic wear in each of the six sextants was given a score of zero to three. A score of zero was defined as no signs of erosive wear on the tooth. A score of one would indicate beginning signs of superficial enamel texture. A score of two was a more noticeable enamel deficiency, or a decrease in hard tissue, that was less than 50% of surface area.
However, a score of three was recognized as a decrease in hard tissue that was more than 50% of the total surface area. After each sextant was scored, each of the six scores are added together. This final score indicated which range of the risk level the patient was categorized in (Bartlett, Ganss, and Lussi, 2008).
A patient with a higher cumulative BEWE score should be given more recommendations for trying to prevent further damage, which could include information on nutrition, identifying any negative habits that would produce erosion, oral hygiene instructions tailored to erosive wear, products containing fluoride, or even dental restorations if needed. A higher score would indicate further monitoring; and a sextant with a score over two should be watched closely for dentin exposure. The BEWE score should be documented in the patient's chart to help monitor dental erosion, and a higher BEWE score indicates that the examination should be repeated more frequently at dental visits. Taking intraoral pictures could help determine changes in scores during examinations, and assist with patient education on erosive wear and documentation. The BEWE or a similar index could help clinicians notice erosive wear, a condition that should be monitored to greatly help manage and stop its progression (Bartlett, Ganss, and Lussi, 2008).
Conclusion
An integral role is played in the diet and how the body is affected. Evidence has shown that an alkaline diet, alkaline water, and intake of the necessary amount of protein played a role in the remineralization of bone. A promotion in health was indicated in the consumption of more fruits, vegetables, and alkaline water, especially in people of an older age group. With advanced age, there is an increased propensity for low-grade acidosis due to the reduced renal capacity. The presence of metabolic acidosis affected the ability of the body to excrete toxins efficiency.
During digestion, reduced digestive tract bacterial load also played a vital role in the increase of blood alkalinity toward the normal upper limit. The factors that increased this change in blood alkalinity were flossing of teeth, good oral hygiene, properly chewed food, and bowel evacuation as soon as the urge for propulsion was felt (Mousa, 2016). The alkaline diet has been proven to result in numerous health benefits such as healthy bones, induce higher osteoblast activity, bone remineralization, preservation of muscle mass, reduction of tumor invasiveness and metastasis, and improvement in the excretion of toxins.
In the general population there was unawareness that tap water was the source with most stringent regulations compared to bottled water. In the author’s water experiment, the conclusion was that most bottled waters were generally more acidic compared to tap water, which had a neutral pH. Alkaline water has also shown positive health benefits by the reduction of congenital heart disease, arteriosclerotic heart disease, cardiovascular disease, cancer incidence, lowered total mortality rates, prevention of osteoporosis, and the protection of pancreatic beta cells (Mousa, 2016).
The use of alkaline water has even demonstrated buffering abilities on stomach acid as well as a therapeutic agent to prevent tissue bound proteins in the pharyngoesophageal tissue. Tissue bound proteins are seen more often in patients with reflux disease which could eventually lead to cancer. The BEWE index was explained for the benefit of dental professionals, to help manage and prevent the effects of an acidic diet on tooth wear.
Natalie Sommers and Katelyn Kent are dental hygiene students at Collin College in Texas.
References
- Barbu A, Neamtu M, Zahan M, Miresan V. (2016). Novel uses of probiotics in human health. Acta Medica Transilvanica, 21(3), 26-30.
- Bartlett D, Ganss C, Lussi A. (2008). Basic erosive wear examination (bewe): a new scoring system for scientific and clinical needs. Clinical Oral Investigations, 12(Suppl 1), 65–68. http://doi.org/10.1007/s00784-007-0181-5.
- Barzel US, Massey LK. (1998). Excess dietary protein can adversely affect bone. The Journal of Nutrition, 128(6), 1051+. Retrieved from http://go.galegroup.com.library.collin.edu/ps/i.do?p=HRCA&sw=w&u=txshracd2497&v=2.1&it=r&id=GALE%7CA20871649&asid=35859ef7d002dcc488c8ab2b3726d9d6.
- Collison D. (2005). Acid alkali balance – the ideal diet. Retrieved from http://www.huntlycentre.com.au/updates/posts/view/118.
- Collison D. (2010). Pral - potential renal acid load a measure of the effect of foods on the ph of the body. Retrieved from http://www.huntlycentre.com.au/updates/posts/view/118.
- Dawson-Hughes B, Harris SS, Ceglia L. (2008). Alkaline diets favor lean tissue mass in older adults. The American journal of clinical nutrition, 87(3), 662-665.
- Kim MJ, Kim HK. (2012). Anti-diabetic effects of electrolyzed reduced water in streptozotocin-induced and genetic diabetic mice. Life Science, 79(24), 2288-2292.
- Koufman JA, Johnston N. (2012). Potential benefits of ph 8.8 alkaline drinking water as an adjunct in the treatment of reflux disease. annals of otology, rhinology & laryngology, 121(7), 431-434.
- Lewis MW. (2010). Costly water: Bottled and Sold: The history behind our obsession with bottled water. Issues In Science & Technology, 27(1), 85-88.
- Martin, L. Protect against the effects of acid wear. (2013). British Dental Journal, 215(9), 477.
- (2016, November 11). Metabolic acidosis. Retrieved from https://medlineplus.gov/ency/article/000335.htm
- Meghji S, Morrison MS, Henderson B, Arnett TR. (2001). pH dependence of bone resorption: Mouse calvarial osteoclasts are activated by acidosis. American Journal of Physiology [Consolidated], 280(1), E112. Retrieved from http://go.galegroup.com.library.collin.edu/ps/i.do?p=HRCA&sw=w&u=txshracd2497&v=2.1&it=r&id=GALE%7CA70421653&asid=e097e71c5242292bfbab416660148fab.
- Mousa HA. (2016). Health effects of alkaline diet and water, reduction of digestive-tract bacterial load, and earthing. Alternative Therapies In Health & Medicine, 22(S1), 24-33.
- Saylor A, Prokopy L, Amberg S. (2011). What's wrong with the tap? examining perceptions of tap water and bottled water at purdue university. Environmental Management, 48(3), 588-601. doi:10.1007/s00267-011-9692-6.
- Schwalfenberg GK. (2012). The alkaline diet: is there evidence that an alkaline ph diet benefits health?. Journal Of Environmental & Public Health, 1-7. doi:10.1155/2012/727630.
- Williams S, Krueger N, Davids M, Kraus D, Kerscher M. (2007). Effect of fluid intake on skin physiology: distinct differences between drinking mineral water and tap water. International Journal Of Cosmetic Science, 29(2), 131-138.
- Wright KF. (2015). Is your drinking water acidic? A comparison of the varied pH of popular bottled waters. Journal of Dental Hygiene (Online), 89, 6-12. Retrieved from: http://library.collin.edu/login?url=http://search.proquest.com.library.collin.edu/docview/ 1788486151?accountid=7969.