Smoking and Periodontitis
There is an abundance of scientific evidence that smoking has an additive effect on the progression of periodontal disease and is detrimental to healing after periodontal therapy.
Cigarette smoking is one of the most preventable sources of morbidity and premature death worldwide. In the United States, smoking is responsible for approximately one in five deaths.1 Smoking prevalence has been on the decline in recent years. In 1963, the per capita consumption was 4,354 cigarettes compared to an estimated 1,979 in 2002, a lower level not seen since the 1940s. However, with the steady decline, smoking still costs the economy more than $150 billion in annual health-care costs and lost productivity, including $75.5 billion in excess medical expenditures. These expenditures include dental costs, since smoking increases the disease progression and complicates the treatment of periodontal diseases.
Women between the ages of 20 and 39 who smoke cigarettes have approximately twice the chance of having periodontal disease or becoming edentulous as do nonsmokers.7 Overall, smoking is probably the single most significant, modifiable risk factor for periodontal diseases. The incidence of periodontitis is 4.9 percent for never smokers, 10.5 percent for former smokers, and 15.6 percent for current smokers.16 The evidence suggests that more than one-half (8.1 million cases) of the chronic periodontitis cases in the United States are attributable to cigarette smoking. There is an abundance of scientific evidence that smoking has an additive effect on the progression of periodontal disease and is detrimental to healing after periodontal therapy. One of the earliest studies to show a relationship between smoking and periodontal health was conducted on Swedish army soldiers.2 The subjects who smoked were shown to be at greater risk for gingivitis, but no differences were noted in bone loss or increased periodontal pocketing. However, another study demonstrated that the alveolar bone height was significantly reduced in smokers compared to nonsmokers.3 Likewise, Haber and Kent demonstrated that smokers were 2.7 times more likely to have moderate to advanced periodontal disease.4 Also, smoking has been shown to significantly increase the risk of tooth loss from periodontal disease.5 The effect appears to be dose-related, with heavy smokers exhibiting a significantly greater risk of tooth loss from periodontal disease compared to nonsmokers and lighter smokers.6
Pathology of smoking and periodontal disease
One hypothesis for the increased periodontal changes noted in smokers is that the periodontal pockets of smokers tend to be more anaerobic compared to nonsmokers.8 An anaerobic environment could conceivably promote the growth of Gram-negative periodontal pathogens in the subgingival plaque. However, the study using Gram staining techniques failed to show a significant difference in the subgingival microflora between smokers and nonsmokers. Since smoking does not appear to significantly change the periodontal microflora, some researchers have suggested that smoking may have an effect on the host response. It has been hypothesized that smoking may alter the host response in two ways: Smoking could impair the normal functions of the host response in neutralizing infection, and À it may alter the host response, resulting in destruction of the surrounding healthy periodontal tissues.
Several studies have shown that the effect of smoking on periodontal tissues may involve both of these processes. For example, smokers tend to have depressed numbers of T-helper lymphocytes, which are important cells of the immune system to regulate cell-mediated immunity and the activity of B lymphocytes.9,10 In addition, the host requires functional neutrophils to deal effectively with bacterial infections. Tobacco smoke has been shown by several studies to have a deleterious effect on various neutrophil functions. For example, smoking has been shown to impair the chemotaxis and phagocytosis of both oral and peripheral neutrophils.11,12 Neutrophils are found in inflammatory lesions, particularly acute lesions, where they concentrate at the site of injury. They are chemically attracted to the site by a process called chemotaxis. Once at the site of injury, neutrophils then engulf (phagocytosis) and kill most microorganisms and neutralize other noxious substances. It has been shown that impaired neutrophil function can contribute to more severe periodontal destruction.13
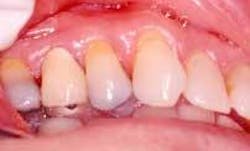
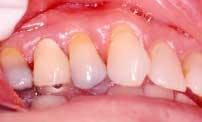
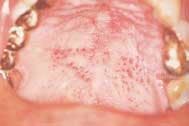
Disease masking is a term that has been applied to describe the appearance of the gingiva associated with chronic smokers.23 Typically, the diseased tissues of smokers tend to have a firmer appearance and less bleeding compared to that of nonsmokers. The term disease masking is used because the vasoconstrictive properties of tobacco smoke hide the inflammatory and destructive changes occurring within the periodontium (See Figures 1a and 1b). The periodontal tissues are compromised by the initial vasoconstriction, resulting in decreased blood flow to the gingiva. This masks the normal early signs of periodontal problems by decreasing gingival inflammation, erythema, and bleeding despite the presence of the disease.
null
null
Signs and symptoms
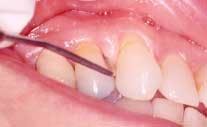
Acute necrotizing ulcerating gingivitis (ANUG) has also been shown to be clearly correlated to smoking, but no cause-and-effect relationship has been demonstrated.14 It is thought that both smoking and ANUG may be the result of underlying anxiety and stress. The condition involves primarily the free gingival margin, the crest of the gingiva, and the interdental papillae. Rarely, the lesions can spread to the soft palate and tonsillar areas, resulting in the condition known as Vincent's angina. ANUG is characterized by punched-out papilla, pronounced gingival erythema, and spontaneous hemorrhage (See Figure 2). Local lymphadenopathy and slight elevation of temperature may also be present. The disease may resolve spontaneously, but treatment usually consists of mechanical debridement and systemic antibiotic treatment.
null
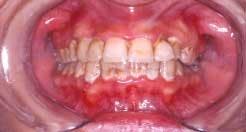
Nicotinic stomatitis, or smoker's palate, is another oral change that is characteristic of smokers. It is characterized by prominent mucous glands with inflammation of the orifices and a diffused erythema, or by a wrinkled, "cobblestone" appearance of the palate often described as a "dried lake bed" effect (See Figure 3). This visual appearance is the result of thickening of the epithelium adjacent to the orifice in response to chronic irritation. The regression of these lesions upon smoking cessation has led many researchers to conclude a cause-and-effect relationship.
null
Etiology
Nicotine is one of the most studied components of tobacco products and the most pharmacologically active compound in tobacco smoke. It has been shown to have various mood-altering effects on its consumers. Nicotine is a poisonous alkaloid found in tobacco smoke and can enter the body by absorption through the oral mucosa and skin or inhalation via the lungs. Nicotine is also highly addictive. Only 2.5 percent of the 34 percent of smokers who attempt to quit smoking are successful.15 Nicotine has numerous detrimental effects on periodontal cells. In vitro, nicotine has been shown to inhibit gingival fibroblast growth and production of fibronectin and collagen, which are necessary building blocks to a healthy periodontium. Nicotine also promotes collagen breakdown.17 Periodontal cells exposed to nicotine have also been shown to have decreased growth and protein content, damaged cell membranes, and atypical shapes.18 Tobacco use has been implicated as a risk factor for alveolar bone loss.19 One hypothesis has been the possible stimulant effect of nicotine on osteoclastic activity, the cells most responsible for bone resorption.20 A study of porcine bone marrow cells found that nicotine is nontoxic to osteoclasts at clinically relevant levels, and appears to stimulate osteoclast differentiation and the resorption of calcium phosphate, the major inorganic component of bone. It is thought that nicotine-modulated osteoclast stimulation may partially explain the increased rapidity of alveolar bone loss and refractory disease incidence in smokers. Additionally, various in vivo modulating factors exist that may exacerbate nicotine's effects on accelerated alveolar bone loss, such as specific subgingival plaque species.21 Nicotine has also been thought to delay apoptosis.22 A delay in cell death has been thought to contribute to tumor production. Also, this delay would allow for osteoclasts to continue the resorptive process longer than their normal life cycle would have permitted. These factors may also contribute to the accelerated alveolar bone loss seen in smokers.
Conclusion
In conclusion, whether it is direct heat from the cigarette, the vasoactive response from nicotine, or a change in the host response to periodontal pathogens, the mechanism by which smoking induces periodontal attachment loss is currently unknown. Smoking has not only been shown to increase the severity of periodontal disease, but also to decrease the response of the gingival tissues to periodontal therapy, resulting in a greater incidence of refractory disease. Obviously, there is a plethora of published information correlating periodontal diseases to both tooth loss and systemic manifestations. These systemic manifestations include increased risk of coronary artery disease; diabetes; osteopenia; and premature, low-birth-weight babies. Further, it has been demonstrated in numerous studies that smoking cessation leads to improved periodontal health and improved response to periodontal therapy, thus improving overall health. Therefore, it would greatly benefit our patients if we, as dental professionals, made a deliberate effort to promote smoking-cessation programs as well as educate our community on the benefits of not smoking.
References
- Trends in tobacco use: American Lung Association Epidemiology. American Lung Association Epidemiology and Statistics Unit Research and Scientific Affairs. June 2003.
- Preber H, Kant T, Bergstrom J. Cigarette smoking, oral hygiene, and periodontal health in Swedish Army conscripts. J Clin Periodontol 1980; 7:106-113.
- Bergstrom J, Eliasson S. Cigarette smoking and alveolar bone height in subjects with a high standard of oral hygiene. J Clin Periodontol 1987; 14:466-469.
- Haber J, Kent R. Cigarette smoking in a periodontal practice. J Periodontol 1992; 63:100-106.
- McGuire MK, Nunn ME. Prognosis vs. actual outcome III. The effectiveness of clinical parameters and IL-1 genotype in accurately predicting tooth survival. J Periodontol 1996; 67:666-674.
- McGuire MK, Nunn ME. Prognosis vs. actual outcome IV. The effectiveness of clinical parameters and IL-1 genotype in accurately predicting prognoses and tooth survival. J Periodontol 1999; 70:49-56.
- Solomon H, Priore R, Bross I. Cigarette smoking and periodontal disease. J Am Dent Assoc 1968; 77:1081.
- Kenney EB, Saxe SR, Bowles RD. The effect of cigarette smoking on anaerobiosis in the oral cavity. J Periodontol 1975; 46:82-85.
- Costbel U, Bross KJ, Reuter C, Ruhle KH, Mattheys H. Alterations in immunoregulatory T cell subsets in cigarette smokers. A phenotypic analysis of bronchoalveolar and blood lymphocytes. Chest 1986; 90:39-44.
- Ginns LC, Golenheim PD, Miller LG. T-lymphocyte subsets in smoking and lung cancer. Analysis of monoclonal antibodies and flow cytometry. Am Rev Respir Dis 1982; 126:265-269.
- Kenney EG, Kraal JH, Saxe SR, Jones J. The effect of cigarette smoke on human oral polymorphonuclear leukocytes. J Periodont Res 1977; 12:227-234.
- Selby C, Drost E, Brown D, Howie S, Mac Nee W. Inhibition of neutrophil adherence and movement by acute cigarette smoke exposure. Exp Lung Res 1992; 18:813-827.
- Page RC. The role of inflammatory mediators in the pathogenesis of periodontal disease. J Periodontol Res 1991; 1(1):4-11.
- Carranza FA. Glickman's Clinical Periodontology. 8th ed. 1996. WB Saunders Co.
- Benowitz NL. Clinical pharmacology of nicotine. Ann Rev Med 1986; 37:21-32.
- Tomar SC, Asma S. Smoking attributable to periodontitis in the United States: findings from NHANES III. J Periodontol 2000; 71:743-751.
- Tipton DA, Dabbous MK. Effects of nicotine on proliferation and extracellular matrix production of human gingival fibroblasts in vitro. J Periodontol 1995; 66:1056-1064.
- Alpar B, Leyhausen G, Saptonik A, Gunay H, Guertsen W. Nicotine-induced alterations in human primary periodontal ligament and gingival fibroblast cultures. Clin Oral Invest 1998; 2:40-46.
- Grossi SG, Genco RJ, Machtei EE. Assessment of risk for periodontal disease II. Risk indicators for alveolar bone loss. J Periodontol 1995; 66:23-29.
- Hennemeyer CL, Scales DK, Hokett SD, Cuenin MF, Peacock ME, Parker MH, Brewer PD, Chuang AH. Nicotine stimulates osteoclast resorption in a porcine marrow cell model. J Periodontol 2003; 74:1440-1446.
- Haber J, Wattles J, Crowley M, Mandell R, Joshipura K, Kent RL. Evidence for cigarette smoking as a major risk factor for periodontitis. J Periodontol 1993; 64:16-23.
- Wright SC, Zhong J, Zheng H, Sarrick JW. Nicotine inhibition of apoptosis suggests a role in tumor promotion. FASEB 1993; 7:1045-1051.
- Bergstrom J, Floderus-Myrhed B. Co-twin control study of the relationship between smoking and some periodontal disease factors. Community Dent Oral Epidemiol 1983; 11:113-116.
Rebecca B. Davis, DDS, MSD
Dr. Davis is an assistant clinical professor at the Case Western Reserve University School of Dentistry. In 2002, the AAP recognized her master's thesis on the relationship between cardiovascular disease and periodontitis. Dr. Davis is in private practice in Lyndhurst, Ohio, specializing in periodontics.
Roger A. Hess, DDS, MBA, MPA
Dr. Hess is an assistant clinical professor at the Case Western Reserve University School of Dentistry. He currently serves as editor and board member for the Ohio Academy of Periodontology. Dr. Hess is in private practice in Lyndhurst, Ohio, specializing in periodontics.
Photos courtesy of Dr. Roger Hess.