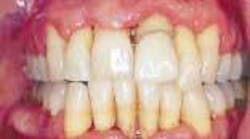
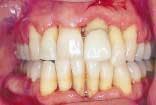
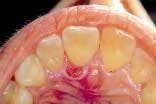
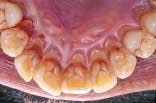
With a focus on the immune response with host modulatory therapy, patients can shift the odds in their favor against periodontal disease.
For years, we believed that poor oral hygiene and age were the primary predictors of periodontal disease in humans. After 20 years of fighting plaque, I can truly appreciate the other side of the imbalance — the host immune response. We have all seen it. Patients will undertake good plaque control, yet they continue to break down. Dr. Ray Williams blames innate risk factors, which include gender, neutrophil function, and genetics.1 We finally have scientific evidence that much of periodontal disease can be attributed to heredity. So how do we incorporate that information into our practices?
One thing is certain; no one wants to feel blamed or guilty about disease. Telling your patient that the problem is genetic often results in a sigh of relief. We still, however, must address oral hygiene and do a thorough root planing. And we must educate our patients on the role of the host response in their disease. Recent research by Dr. Roy Page indicates that genetics are responsible for 50 percent of patients' susceptibility. The good news to our patients is that we can adjust the way our body reacts to pathogens.1
We now have a way to address the host response through host modulatory therapy (HMT). I routinely tell patients, "Up until recently, there was nothing we could do about the genetic component of periodontal disease. But you now have the ability to make a difference in your mouth." Conveying this information to patients is a form of empowerment.
At this time, there is only one HMT available. Periostat® (doxycycline hyclate) is a 20 mg tablet. The original concept for Periostat was developed by Dr. L.M. Golub.2 When used as a subantimicrobial dosage, Periostat decreases the production of harmful enzymes, while having no effect on the level of periodontal microorganisms.3-5 By decreasing the activity of these enzymes there is a reduction in tissue and alveolar bone break down.6,7 When compared to scaling and root planing alone, mechanical therapy combined with Periostat results in statistically significant reduction in pocket depths, bleeding, better attachment, and prevention of disease progression.8
null
null
In private practice, this translates to healthier and more attractive soft tissue. The healthier soft tissue can be especially helpful in cosmetic dentistry, as well as for making impressions for anterior crown and veneers less stressful.
In my practice, we have more than 1,000 patients on HMT and have had very few negative side effects. I generally give a prescription of Periostat along with the patient's scaling and root planing for a length of three to six months, depending on the severity of the disease. The patient is then requested to return for a follow-up visit two months after SRP. After this re-evaluation, I determine how long the patient should continue on host modulation therapy with Periostat. Patients with risk factors — such as smoking, diabetes, stress, poor nutrition, history of periodontal disease, or family history of periodontal disease — generally stay on the drug for a period of six to nine months with re-evaluations at three-month maintenance intervals.
At any time during this evaluation process, I will recommend surgery if I feel the patient needs it. For long-term treatment, I re-evaluate the patient. Each patient is a unique case, and many factors determine the continued use of the drug. Periostat allows me to control the "non-microbial risk factors" or host response of the patient and, therefore, allows me to stabilize tissue and bone destruction while I continue my therapy. It is not a substitute for my treatments; rather, it enhances what I do in everyday clinical practice.
In fact, it is one of the few therapies that continually brings positive feedback from patients, not common in a periodontal practice. Patients call us to report they feel more confident, their teeth are not as loose or sensitive, and they have noticed a decrease in bleeding. When patients feel like they are playing an active role in their care, you tend to have a better outcome. Without nagging, we reward the good behavior (home care) and encourage Periostat to address the destructive enzymes produced as a result of the host inflammatory response. Host modulatory therapy is especially important for our more high-risk patients.
The American Academy of Periodontology (AAP) recognizes that periodontal tissue destruction is primarily due to the host response. In addition, the academy acknowledges that host modulatory therapy may be beneficial in patients with increased susceptibility to disease progression.9
An informational paper by the academy, "Modulation of the Host Response in Periodontal Therapy," states, "... there are situations in which conventional therapy does not always achieve the desired clinical outcome. Certain patients possess non-microbial risk factors which are difficult to reduce or eliminate (e.g., smoking, diabetes) or are beyond the clinician's ability to control (e.g., genetic predisposition)."9
In my practice, I also group HIV patients and even menopausal women into this high-risk category. These patients have an altered host response and plaque control alone does not adequately treat these difficult cases. By combining several modalities of care, we can achieve better control and look for improvement over time. Patients with a long-span bridge can also benefit from HMT. Cosmetic dentistry is a big investment, which needs to be protected as much as possible. Periostat therapy is one more way to maintain good tissue health over time.
Another argument for using HMT in our practice comes from the exciting new awareness of the correlation between oral health and overall general health. Recent studies by Dr. Robert Genco's group have evaluated the association between subgingival microorganisms and myocardial infarction.10 A high correlation was discovered between specific pathogenic bacteria and myocardial infarction. An increasing number of studies support an association between periodontal disease and cardiovascular diseases.11-14
Periodontal disease has been associated with systemic inflammatory mediators, such as C-reactive protein (CRP), which are positively associated with acute myocardial infarction and sudden cardiac death.15-17 This inflammatory marker (CRP) has been found to be a predictor of increased risk for cardiovascular disease (CVD) and is also related to disease level in periodontitis.18,19 In a more recent study published in the New England Journal of Medicine, it was concluded that the CRP level is a stronger predictor of cardiovascular events than cholesterol (low-density lipoprotein, LDL).20
Interestingly, a more recent finding by Dr. David Brown of Beth Israel Medical Center in New York found that "Periostat significantly reduced levels of the inflammatory marker (CRP) in patients hospitalized for acute coronary syndrome (ACS) and the study suggests that Periostat may have a protective benefit in patients who are at risk of cardiovascular disease."21 There is an association between periodontal disease, CRP, and an increased risk of cardiovascular disease. Once again, we do not want to use this information to scare or intimidate patients, but to enlighten and educate.
In summary, in a private practice we have to not only look at the research, but how it affects our everyday lives and how we practice. I want to do everything I can to get the absolute best results with my patients. The research is solid and evidence-based, but, more importantly, the clinical results we see are obvious. By treating our patients with compassion rather than intimidation, we are going to have a healthier and happier outcome. Host modulatory therapy with Periostat gives us one more weapon in the war against periodontal disease. As far as I am concerned, I want to stack the odds as high in my favor as possible.
References
- Williams RC. Periodontal disease: The emergence of a new paradigm. Comp Cont Educ Dent 1999; 19 (special issue):4-10.
- Golub LM, Wolff M, Lee HM, et al. Further evidence that tetracyclines inhibit collagenase activity in human crevicular fluid and from other mammalian sources. J Periodontal Res. 1985; 20:12-23.
- Golub LM, et al. Modulation of the host response in the treatment of periodontitis. Dentistry Today 1998; 17(10):102-109.
- Thomas JG, et al. Long-term use of subantimicrobial dose doxycycline does not lead to changes in antimicrobial susceptibility. J Periodontol 2000; 71(9):1472-1483.
- Walker C, et al. Long-term treatment with subantimicrobial dose doxycycline exerts no antimicrobial effect on the subgingival microflora associated with adult periodontitis. J Periodontol 2000; 71(9):1465-1471.
- Golub LM, et al. Tetracyclines inhibit connective tissue breakdown by multiple nonantimicrobial mechanisms. Adv Dent Res 1998; 12:12-26.
- Vernillo AT, Rifkin BR. Effects of tetracyclines on bone metabolism. Adv Den Res 1998; 12:56-62.
- Caton J, et al. Treatment with subantimicrobial dose doxycycline improves the efficacy of scaling and root planing in patients with adult periodontitis. J Periodontol 2000 (in press).
- Academy Report, Informational Paper, Modulation of the Host Response in Periodontal Therapy. J Periodontol 2002; 73:460-470.
- Genco RJ, Wu T, Gross S, Faulkner K, Zambon JJ, Trevisan M. Periodontal microflora related to the risk of myocardial infarction: a case-control study (abstract 2811). J Dent Res 1999; 78 (special issue):457.
- Beck JD, Offenbacher S. The association between periodontal diseases and cardiovascular diseases: A state-of-the-science review.
- DeNardin E. The role of inflammatory and immunological mediators in periodontitis and cardiovascular disease. Ann Periodontol 2001; 6(1):30-40.
- Emingil G, et al. Association between periodontal disease and acute myocardial infarction. J Periodontol 2000; 71(12):1882-1886.
- Arbes SJ, et al. Association between extent of periodontal attachment loss and self-reported history of heart attack: An analysis of NHANES III data. J Dent Res 1999; 78(1):1777-1782.
- Liuzzo G, et al. The prognostic value of C-reactive protein and serum amyloid A protein in severe unstable angina. N Engl J Med 1994; 331:417-424.
- Thompson SG, et al. Hemostatic factors and the risk of myocardial infarction or sudden death in patients with angina pectoris. N Engl J Med 1995; 332:635-641.
- Tracy RP, et al. Relationship of C-reactive protein to risk of cardiovascular disease in the elderly: Results from the Cardiovascular Health Study and the Rural Health Promotion Project. Atheroscler Thromb Vasc Biol 1997; 17:1121-1127.
- Noack B, et al. Periodontal infections contribute to elevated systemic C-reactive protein level. J Periodontol 2001; 72(9):1221-1227.
- Elgharib N, Chi DS, Younis W, Wehbe S, Krishnaswamy G. C-reactive protein as a novel biomarker. Reactant can flag atherosclerosis and help predict cardiac events. Postgrad Med. Dec. 2003; 114(6):39-44.
- Ridker PM, et al. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med 2002; 347(20):1557-1565.
- Brown D, et al. Clinical and Biochemical Results of the Metalloproteinase Inhibition with Low-Dose Doxycycline to Prevent Acute Coronary Syndromes (MIDAS) Pilot Trial. Circulation 2002; 106: (spec issue 19); Abstract 2253: IT - 455.
Laura D. Braswell, DDS
Dr. Braswell has more than 20 years of dental experience, including clinical research and a faculty appointment at Emory University in the department of periodontology. She owns Buckhead Periodontics in Atlanta, Ga., and lectures on drug therapy, periodontology surgery, and zoo dentistry.