Bone Regeneration in periodontal defects, ridge augmentation, and in preparation for dental implants
We have come a long way with the ability to replace lost alveolar bone so that diseased teeth may be saved or lost teeth may be replaced by means of dental implants.
Written By: Kathleen A. Stambaugh, DDS
Tremendous strides have been made in the ability to create lost bone resulting from periodontitis or trauma. Various surgical techniques have been utilized over the years and there are many successful procedures to choose from. In general, reconstruction of bone is optimal when autogenous bone is used for grafting, likely due in part to the quantity of bone morphogenetic protein that is present in fresh bone obtained from the patient. Several decades ago, the practice of utilizing autogenous (from the patient) hip marrow grafts were in vogue.1 This practice however, resulted in significant morbidity in some cases, and many times the outcome was root resorption around teeth. The bone marrow was thought to be “too fresh.” Hip marrow is still utilized today for ridge augmentation by some clinicians, as well as anterior tibia grafts. The tibia grafts have moderate to severe morbidity, as they can result in tibia fracture if the graft is taken too low, or the bone is otherwise compromised.
Regenerating bone in periodontal defects around teeth or in extraction sockets to plan for future implants differs somewhat in technique. Bone regeneration in periodontal defects is very technique-sensitive. It is dependent upon several factors, including incision decision, complete debridement of the bone defect and root, skill of the clinician, shape and size of the defect, compliance of the patient including plaque control and smoking habits, health of the patient, and type of graft material used. Autogenous bone is considered the best and most predictable bone grafting material. But due to limitations in harvesting sufficient quantities, it is frequently mixed with a synthetic bone-like material, allograft or xenograft.2 Demineralized, freeze-dried bone allograft (DFDBA) has been a popular and effective material over the past several decades, resulting in mean defect fill of 60 to 65 percent in a number of studies. The database for DFDBA is the most extensive of the graft materials with large numbers of human blocks analyzed over the years. Although safety has been demonstrated through numerous studies, patient concern still exists regarding the implanting of another individual’s tissue into their bodies, particularly as it may pertain to HIV transmission. The probability that a particular brand of DFDBA might contain HIV is 1 in 2.8 billion.3 Despite the safety of DFDBA, patient concerns of potential disease transmission have supported development of synthetic graft materials which can also be used to promote defect fill. These materials may also cost less than DFDBA and are, therefore, attractive to clinicians.
One such synthetic bone graft particulate is PerioGlas.® This is a bioactive glass material (Bioglass®) that is composed of elements found naturally in bone (Ca, P, Si, O, and Na). The product is supplied in particulate form with the particle size ranging from 90 to 710 microns. When implanted into living tissue, this bioactive glass undergoes a surface reaction resulting in the formation of a calcium phosphate layer. This layer is equivalent in structure and composition to the hydroxyapatite in bone mineral and provides the bonding interface between bone and soft tissue. Another Bioglass® material that is often used is BioGran.® Bioglass® materials may be used in the following applications to generate new bone.
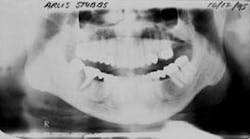
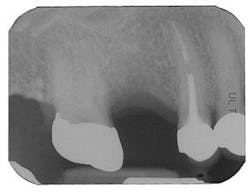
Figure 1 - Panoramic of hopeless teeth under fixed bridge
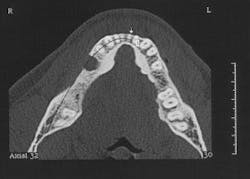
Figure 2 - CT scan showing large bone defect
Figure 2a - CT scan showing large bone defect
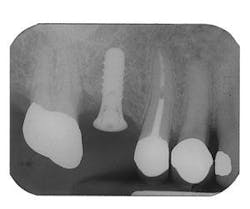
Figure 3 - Ridge augmentation with Perioglas and Titanium-reinforced Goretex membrane
Figure 4 - Bone-grafted ridge prior to implant placement
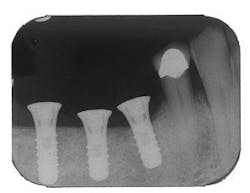
Figure 5 - Placement of three ITI implants
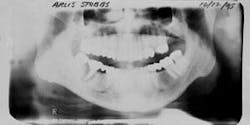
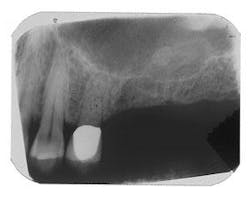
Figure 6 - Same patient with hopeless Tooth No. 3
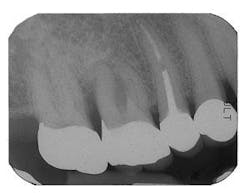
Figure 7 - Extraction Tooth No. 3 / Perioglas graft
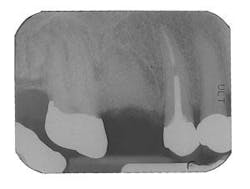
Figure 8 - Healed bone graft Tooth No. 3
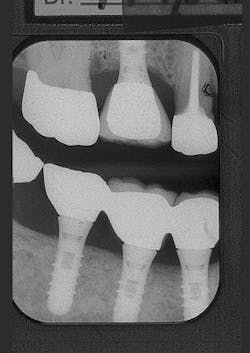
Figure 9 - ITI implant placement Tooth No. 3
Figure 10 - Restored implants
Figure 11 - Patient with insufficient bone below the sinus for implants
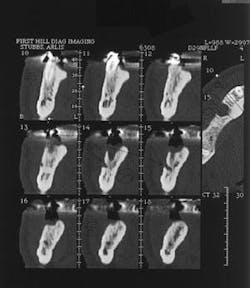
Figure 12 - CT scan demonstrating insufficient bone for implants (SimPlant software)
Figure 13 - Simultaneous sinus lift using autogenous bone plus Perioglas and ITI implant placement
Periodontal defects
A full thickness flap is opened, conserving thickness and quantity of gingiva. The periodontal defect is debrided thoroughly, with magnification and confirmation that no root fractures are present. Use of periodontal endoscopy is particularly helpful during debridement. All soft tissue tags are removed. Root planing burs are generally used. Tetracycline may be used to condition the root. Intramarrow penetration of the defect is performed using a ¼ or ½ round bur and bleeding is established. Depending upon the shape and location of the defect, Emdogain® enamel matrix protein is frequently used prior to introduction of the Bioglass® material. Bioglass® is mixed with tetracycline HCl powder in a 4:1 ratio and this is hydrated with sterile saline at least 15 minutes before introduction into the defect. The material is layered in, while tapping gently over a sterile, damp, unfilled 2 X 2 gauze. It is important not to overcondense the material; presence of blood must be established. With large defects or defects involving loss of the buccal or lingual/palatal plate, it may be necessary to also place a Goretex® membrane over the defect which has been filled with the graft material. After filling the defect, the flaps are sutured obtaining primary closure using a Goretex® or other nonbraided suture. A dressing is placed to protect the site and the patient is instructed to avoid trauma and smoking. Chlorhexidine is prescribed as an oral rinse twice daily and doxycycline 100 mg is prescribed for 10 days (one per day) to enhance bone regeneration.
Careful postoperative care and patient compliance are critical. Results are generally monitored radiographically and clinical probing is not done in this site for six to nine months. It is recommended that periodontal maintenance procedures be completed every three months in the periodontist’s office for at least six to nine months until site stability may be confirmed.
Extraction sites
If an extraction is contemplated in an area that will involve future tooth replacement, it is important to consider bone preservation and regeneration at the extraction site. This is necessary to create sufficient bone for a future dental implant, or to preserve esthetics of the ridge if a fixed bridge will be considered. The regenerative capacity of the body is optimal at the time of extraction, so if the site may be thoroughly debrided without concern of leaving residual infection, it is generally best to graft at the time of extraction. The technique involves atraumatically removing the tooth, using a periotome if necessary to preserve as much socket bone as possible. In endodontically treated molars, it is frequently advisable to section the tooth first, so that roots may be removed without trauma and loss of bone. The socket is debrided carefully, removing all tissue tags; a large round bur is used in the socket as an aid in this process. Intramarrow penetration is established and a mixture of Bioglass® material (prepared as previously described) is gently layered into the defect, confirming the presence of blood. Collagen or a connective tissue graft is placed over the graft and the area closed if primary closure is not possible. The area may be protected by a periodontal dressing (if stability may be achieved) or a temporary removable treatment partial (“flipper”). It is important to avoid trauma and pressure over the graft site for several weeks. The site is monitored with a post-bone graft radiograph and again at six to eight months to evaluate the site for a dental implant if that is the intended treatment plan. In most cases, a CT scan is then obtained in order to obtain three-dimensional information on the quantity and quality of bone prior to implant placement.
Sinus lift in preparation for dental implants
Bioglass® may also be used (preferably in combination with autogenous bone) in the posterior maxilla if the quantity of bone is insufficient for dental implants. One technique involves the use of osteotomes. A full thickness flap is raised. A 4 mm trephine is used at slow speed to gently separate a core of bone which may be elevated. It is important to perform this very carefully to avoid perforating the sinus membrane. Once mobility is established, the core is gently elevated with a series of osteotomes, placing graft material and gently elevating. Sufficient elevation and augmentation are confirmed radiographically and primary closure is achieved. The area is reevaluated for implant placement eight to nine months following the graft. Sufficient regeneration is typically confirmed by a CT scan. This procedure may sometimes be utilized simultaneously with implant placement if there is sufficient bone to stabilize the implant. A healing period of eight to nine months is observed prior to implant loading.
Ridge augmentation prior to dental implants
At times, when teeth are lost due to periodontitis, large defects in the alveolar bone involving the loss of one or more socket walls results, thus compromising the notion of dental implants. In advanced defects such as this, it may be necessary to expand the ridge buccolingually as well as coronally. In these cases, it is best to use autogenous bone or combine the Bioglass® material with autogenous bone. Several sources of autogenous bone may be utilized: procurement of osseous coagulum during osteoplasty or reduction of tori, collecting cores of bone using trephines and then milling these in a bone mill, block grafts from the chin or ramus, or procuring from sites such as the ascending ramus or ridge using a bone-scraping collection device. A full-thickness flap is elevated in the region to be augmented. It is necessary to extend the flap sufficiently to obtain primary closure over the augmented site. Debridement of the defect is accomplished and confirmed with a large round bur. A titanium-reinforced Goretex® membrane is positioned over the defect as a “tent.” After filling the defect with graft material, the membrane is fixed in place with tacks to ensure nonmobility of the membrane. The flap is closed over the membrane with primary closure, which sometimes requires cut-back incisions or periosteal incisions. A Goretex® suture is used, which may remain in place until Goretex® membrane removal in eight to 12 weeks. The site is reevaluated for suitability of implants nine months later, at which point a CT scan is obtained.
In addition to bioactive glasses as described above, DFDBA, and autogenous bone, another material that has been used successfully for bone regeneration is PepGen P-15™. This product is bovine-derived hydroxylapatite, which contains P-15, a synthetic short chain peptide. P-15 is a biomimetic of a cell-binding region of Type I collagen. PepGen P-15™ is manufactured as radiopaque, rounded particles ranging in size from 250 to 420 microns. It is also supplied as a flowable, gel-like material dispensed from a syringe, and as a putty which can be molded. A multi-center human study compared periodontal bone defect fill using PepGen P-15™, DFDBA (the benchmark in nonautogenous bone graft materials), and open surgical debridement alone. PepGen P-15 was successful 87 percent of the time in patients having a greater than 50 percent defect fill, compared to DFDBA being successful 58 percent of the time in patients having a greater than 50 percent defect fill.
In summary, we have come a long way with the ability to replace lost alveolar bone so that diseased teeth may be saved or lost teeth may be replaced by means of dental implants. Several graft materials may be utilized for this purpose. Research is limited for materials other than autogenous bone or DFDBA. In order of preference - in terms of predictability and success - some graft choices include the following:
• Autogenous bone or combination of autogenous and others listed
• DFDBA (Demineralized freeze-dried bone allograft) or PepGen P-15
• Bioactive glasses such as PerioGlas®, BioGran®, or Bioplant®
If a careful, well-designed surgical technique is used, and the patient is compliant with detailed postoperative instructions including absence of smoking, significant regeneration of lost alveolar bone may be achieved. Although autogenous bone is the most predictable graft material, the morbidity associated with procurement may steer the surgeon toward the selection of graft products that do not involve secondary or time-consuming surgical harvesting procedures.
References
1 Cushing M. Autogenous red marrow grafts: Potential for induction of osteogenesis. J Periodontol 1969; 40:492-497.
2 Mellonig JT. Freeze-dried bone allografts in periodontal reconstructive surgery. Dent Clin An Am 1991; 35:505-520.
3 Russo R, Scarborough N. Inactivation of viruses in demineralized bone matrix. FDA Workshop on Tissue for Transplantation and Reproductive Tissue. June 20-21, 1995, Bethesda, MD.
Kathleen A. Stambaugh, DDS
Dr. Stambaugh, a board-certified periodontist, currently serves as president of the Western Society of Periodontology. She is a diplomate of the American Board of Periodontology, and maintains a private practice in Burlington, Wash. You may contact her at [email protected].