Cervical Screening Awareness Week (CSAW) was June 15-21. The purpose of the week is to focus on the significance of cervical screening as a way to prevent cervical cancer. New guidelines were released in 2012 by the American Cancer Society (ACS), American Society for Colposcopy, Cervical Pathology (ASCCP), and American Society for Clinical Pathology (ASCP).1A chart with the guidelines is available.2 This article will review the topic of cervical cancer.
Cervical cancer is a gynecologic cancer, usually diagnosed in its early stages, thanks to the Pap test. Cervical cancer is preventable due to screening tests such as the Pap test, and to the human papillomavirus (HPV) vaccine. As with any cancer, cervical cancer is treatable with great success if diagnosed at an early stage, and is associated with long survival and good quality of life. A fact sheet is available that outlines cervical cancer, how to prevent it, risk factors, and symptoms.3
Per the 2012 guidelines, the American Cancer Society (ACS) no longer recommends that women get a Pap test every year.1 They recommend every three years until age 65, except for women who have been diagnosed with cervical pre-cancer. According to the ACS, this group should continue screening with the Pap test. The major exceptions are those women at high risk, such as those with HIV infection, organ transplant, or exposure to the drug DES. Those in the high risk group should seek counsel from their medical professional, per the ACS.
On June 15, a researcher from Keele University in the United Kingdom stated that there is a need for a critical review of cervical screening guidelines.4 The main impetus for this suggestion is to encourage more older women to be routinely screened.
Dr. Sue Sherman states: “This review suggests that older women not getting themselves screened for cervical cancer has become a significant contributor to the number contracting the disease. Despite all the attention on younger women – in part due to the Jade Goody effect – 20 per cent of new diagnoses and nearly 50 per cent of cervical cancer deaths occur in women over the age of 65. We need to change the perception of cervical cancer so it is thought of just like breast and bowel cancer – that it can affect women well into old age.”5
The Jade Goody effect was brought about by the death of a UK celebrity, Jade Goody, at an early age from cervical cancer.6
Another looming issue in cervical cancer is the sexually transmitted human papilloma virus (HPV). There is a test that can be administered to detect infections that can initiate cell alterations and cancer. HPV infections are common, and most types HPV infections do not cause cancer (such as HPV types 6 and 11), but may cause warts. However, about 12 strains of HPV can cause cancer, the most virulent being HPV types 16 and 18.7
In fact, most cases of cervical cancer are HPV related, about 70% caused by HPV 16 and 18, and HPV is implicated in some anal, oropharyngeal, vulvar, and penile cancers.8 The HPV test may be used along with a Pap test, or to help physicians in their decision of how to treat women who have an abnormal Pap test. For more information on the HPV test, visit this site.9 Also, view a good flow chart about patient management of HPV here.
Regarding prevention, the Food and Drug Administration (FDA) has approved three vaccines to prevent HPV infection: Gardasil, Gardasil 9, and Cervarix. These vaccines provide strong protection against new HPV infections, but they are not effective at treating established HPV infections or disease caused by HPV.11 A new study showed that a single dose of the HPV vaccine Cervarix appears to be as effective in preventing certain HPV infections as three doses.12
This is good news as the vaccines are costly. Diane Harper, MD, PhD, said, “Nearly two-thirds of people who get HPV vaccines do not get all three doses in a timely manner.”
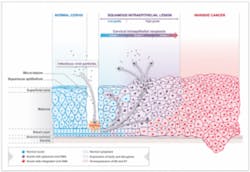
Dr. Harper is involved in some exciting additional research. Genticel, a French biotechnology company and developer of innovative immunotherapies to prevent cancers caused by the human papillomavirus (HPV), recently announced that the FDA cleared Genticel’s investigational new drug (IND) application to conduct a phase 1 clinical study in the United States. The GTL001 drug, known in Europe as ProCervix, will be used in patients infected with HPV16 and/or 18 — the two HPV types responsible for 70% of cervical cancer cases. Dr. Harper will be the principal investigator.13 Today, no treatment options are available for the 93 million women worldwide infected with HPV16 and/or 18 who have not yet developed high-grade lesions or cervical cancer.13
I encourage you to examine the above chart, “HPV Disease Progression,” in more detail at the Genticel website here.
A new study stated that most, if not all, cervical cancers are caused by the HPV infection.15 The researchers studied tissue specimens from four distinct groups: healthy patients; patients with early complications due to HPV infection; patients with advanced complications due to HPV infection; and those with cervical cancer. Den Boon stated that these four groups enabled researchers to study the complete progression from viral infection to cancer.15 The researchers also found that estrogen receptors virtually disappear in cervical cancer tumors.15 These findings shed new light on how cervical cancer should be managed and disease progression controlled.
It is important to educate patients, as well as ourselves, to increase awareness and knowledge about HPV, its relationship with cancer, and the HPV vaccine.
References
- Screening Guidelines for the Prevention and Early Detection of Cervical Cancer. Published online March 14, 2012 in CA: A Cancer Journal for Clinicians. First author: Debbie Saslow, PhD, American Cancer Society, Atlanta, Ga. http://www.cancer.org/cancer/news/new-screening-guidelines-for-cervical-cancer.
- CDC Cervical Cancer Screening Guidelines. www.cdc.gov/cancer/cervical/pdf/guidelines.pdf.
- http://www.cdc.gov/cancer/cervical/pdf/cervical_facts.pdf.
- Keele University Press Release. Call for urgent change to cervical cancer risk perception in older women. https://www.keele.ac.uk/pressreleases/2015/callforurgentchangetocervicalcancerriskperceptioninolderwomen.html.
- Sherman SM, Castanon A, Moss E, Redman CWE. Cervical cancer is not just a young woman’s disease. BMJ, 2015; 350 doi. http://dx.doi.org/10.1136/bmj.h2729. (Published 15 June 2015).
- Marlow LA, Sangha A, Patnick J, and Waller J. The Jade Goody Effect: whose cervical screening decisions were influenced by her story? J Med Screen. 2012 Dec;19(4):184-8. doi: 10.1258/jms.2012.012095. Epub 2012 Dec 27.
- http://www.cancer.gov/about-cancer/causes-prevention/risk/infectious-agents/hpv-fact-sheet.
- Winer RL, Hughes JP, Feng Q, et al. Condom use and the risk of genital human papillomavirus infection in young women. New England Journal of Medicine 2006; 354 (25): 2645–2654.
- http://www.thehpvtest.com/hcp/.
- http://www.thehpvtest.com/hcp/data/hcp-treatment-algorithms/.
- Schiller JT, Castellsague X, Garland SM. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine 2012; 30 Suppl 5:F123-138.
- Kreimer AR, Struyf F, Del Rosario-Raymundo MR, et al. Efficacy of fewer than three doses of an HPV-16/18 AS04-adjuvanted vaccine: combined analysis of data from the Costa Rica Vaccine and PATRICIA trials. The Lancet Oncology. Lancet Online, June 10, 2015. http://dx.doi.org/10.1016/S1470-2045(15)00047-9.
- Genticel’s GTL001 phase 1 clinical study of HPV therapeutic vaccine granted FDA clearance. June 16, 2015. http://www.news-medical.net/news/20150616/Genticele28099s-GTL001-phase-1-clinical-study-of-HPV-therapeutic-vaccine-granted-FDA-clearance.aspx.
- den Boon JA, Pyeon D, Wang SS, et al. Molecular transitions from papillomavirus infection to cervical precancer and cancer: Role of stromal estrogen receptor signaling. PNAS 2015; published ahead of print June 8, 2015, doi:10.1073/pnas.1509322112.