Medical advances: Pharmaceuticals and diagnostics
Developments in medicine are happening at lightning speed. While most of us deal primarily in oral health, it is vital that we are aware of general changes in the rest of the body, such as the overuse of antibiotics and antibiotic resistance and their major global health and economic repercussions. This article will review some recent advances in the medical arena.
New blood test can determine if infection is viral or bacterial
Researchers have discovered a blood test that can rapidly assist physicians in determining if an infection is bacterial or viral. The physicians will then have the capability of prescribing antibiotics more accurately, and only for bacterial infections. (1) The study was a large, multicenter, prospective clinical study that confirms the ability of the ImmunoXpert in-vitro diagnostic blood test to determine whether the infection is bacterial or viral. Prescribing antibiotics for viral infections not only increases health care costs, but also the probability of antibiotic resistance.
Regulation of compounding pharmacies
Compounded bio-identical hormone replacement therapy (BHRT) is in the news again, with mixed opinions on its use. Compounded BHRT is comprised of natural or bio-identical hormones, which are combined in different mixtures, and then placed into topical creams, gels, lotions, tablets, and suppositories. (2) The FDA does not approve these hormones, as they are created especially for each patient. Why is this important? With no FDA oversight, these preparations could be ineffective, harmful, contaminated, or otherwise problematic. Under the new Compounding Quality Act, the FDA has new controls intended to make specialty drugs safer for patients. (3) This is important, as compounding pharmacies, if not answerable to anyone, could produce pharmaceuticals that are harmful. In once instance, more than 60 people were injected with fungus-contaminated steroid medications from a compounding pharmacy while being treated for back and joint pain. In fact, one author stated that “Without federal protection, women who use compounded BHT are risking their health.” (4) While these incidents are more the exception than the rule, become educated on this issue and make intelligent decisions.
READ MORE |Early studies for new painkiller show long-lasting effects
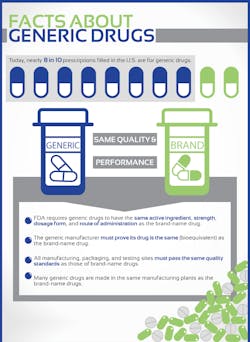
Generic or brand-name?
Another sometimes-confusing issue is the use of brand-name versus generic drugs. Most medications these days are generic, as they are much less expensive. Developing a new prescription pharmaceutical that has marketing approval takes many years, and is likely to cost $2.6 million. (5) According to Generic Drug Savings in The U.S., generic medications have saved Americans one trillion dollars in health care savings in the last ten years, translating to more than $1 billion in savings every other day. (6) Generic drugs are very similar, albeit not exactly like, brand-name drugs. They can be used except in rare cases. Using NTI (narrow therapeutic index) drugs can be complicated because the blood concentrations needed to achieve a therapeutic dose and the concentrations that will cause harm are very close together. (7) For more information, visit the FDA’s Understanding Generic Drugs webpage. (7)
Check out Medical advances: The tech sector for more advancements!
References
1. Oved K, Cohen A, Boico O, et al. A novel host-proteome signature for distinguishing between acute bacterial and viral infections. PLoS One. 2015;10:e0120012. doi: 10.1371/journal.pone.0120012. http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0120012.
2. Menopause glossary. The North American Menopause Society. http://www.menopause.org/for-women/menopause-glossary#C. Accessed June 2, 2015.
3. Compounding. U.S. Food and Drug Administration. http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/PharmacyCompounding/default.htm. Updated May 26, 2015. Accessed June 2, 2015.
4. Ramin CJ. The hormone hoax thousands fall for. More. http://www.more.com/health/wellness/hormone-hoax-thousands-fall-0. Accessed June 2, 2015.
5. DiMasi JA, Hansenb RW, Grabowskic HG. The price of innovation: New estimates of drug development costs. J Health Econ. 2003;22:151-85.
6. Generic Pharmaceutical Association. Generic Drug Savings In The U.S. (Fourth Annual Edition: 2012). http://www.ahipcoverage.com/wp-content/uploads/2012/08/2012-GPHA-IMS-GENERIC-SAVINGS-STUDY.pdf. Accessed June 2, 2015.
7. Pope ND. Generic substitution of narrow therapeutic index drugs. US Pharm. 2009; 34(Generic Drug Review suppl):12-19. http://www.uspharmacist.com/content/s/78/c/13854/#sthash.xE45A29b.dpuf.