Virucidal activity of oral rinses against SARS-CoV-2
Abstract
Background: The aim of this review is to assess the efficacies of different chemical agents on reducing the oral virucidal load of the SARS-CoV-2 virus responsible for the COVID-19 pandemic.
Types of studies reviewed: An electronic search was conducted using Medline (ProQuest), PubMed, and Google Scholar. Twenty-one articles were selected and reviewed for this study, assessing the virucidal efficacy of agents against the SARS-CoV-2 virus in the mouth.
Results: Given the recency of the SARS-CoV-2 virus, research being performed on mouthrinses is still being conducted and conclusions are still being made. The methods used in these studies vary between mostly in vitro and a few in vivo studies. From this review, it is clear that hydrogen peroxide, povidone-iodine (PVP-I), and chlorhexidine all hold great promise against SARS-CoV-2 in the mouth.
Practical implications: A mouthrinse that can reduce the viral load of SARS-CoV-2 will be very useful for dental practitioners to use as a preprocedural agent to reduce viral transmission during practice.
Key words: SARS-CoV-2, COVID-19, mouthwash, chlorhexidine, povidone-iodine, hydrogen peroxide
Introduction
The COVID-19 pandemic that nations around the world are currently facing has had an enormous impact on the health and well-being of all individuals and the stability of entire economic systems. The rapid spread and transmission mechanism of the SARS-CoV-2 virus have left people struggling to keep up with diagnoses and treatments. For this reason, the search for a cure or a method to slow the spread of the virus is being heavily researched at this time. Since research and development for a COVID-19 vaccine may take a considerable amount of time, a focused response to transmission-based precautions, such as mouthrinses, will be very important to inhibit the spread of the virus.1
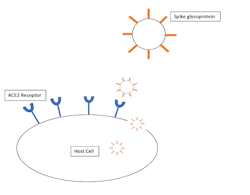
Additionally, it has been observed that the angiotensin-converting enzyme 2 (ACE2) receptor in epithelial cells is a key target for the SARS-CoV-2 virus (figure 1).6,7 However, the expression of ACE2 has been found to be “higher in salivary glands than lungs,” which is a good indication that the mouth, where the salivary glands are located, may be a major replication site for the virus.4 Consequently, increasingly more research is being performed on mouthwashes that may be able to decrease the viral loads in these areas to reduce viral spread, especially as a precaution before procedures of the mouth and throat.
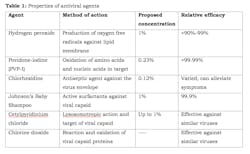
Researchers are currently looking at various formulations of mouthwashes that may be able to reduce the viral load of SARS-CoV-2 in the mouth and nasopharynx. The potential mouthwash must target some aspect of the virus, such as destroying or making holes in the protective lipid envelope.3 Mouthwash formulations that are being considered, but have not been directly compared yet, are hydrogen peroxide, povidone-iodine (PVP-I) and chlorhexidine. Two other significant mouthwash formulations, Johnson’s Baby Shampoo and cetylpyridinium chloride, are also in the beginning phases of research. A general summary of each is outlined in Table 1.
An electronic search using Medline (ProQuest), PubMed, and Google Scholar was performed to assess the literature on completed experiments testing the virucidal activity of mouthwashes containing these three chemicals. Terms that were used for this search included, but were not limited to, “mouthwash,” “hydrogen peroxide,” “povidone-iodine,” “chlorhexidine,” “SARS,” “COVID-19.” Twenty-one articles were selected and compared to evaluate the research that has been performed regarding the virucidal activity of these three agents in the form of a mouthrinse.
Hydrogen peroxide
Hydrogen peroxide is an oxidizing agent that can destroy lipid membranes by producing oxygen free radicals.3 Additionally, hydrogen peroxide is a very cost-effective product, so it has a lot of potential if it can be manipulated to contribute to destroying SARS-CoV-2 in the mouth. For this reason, 1.5% hydrogen peroxide has been included in the current temporary American Dental Association (ADA) guidelines to reduce the risk of COVID-19 transmission during dental treatments.8
According to an in vitro study, the typical “3% solution [of hydrogen peroxide] should be diluted up to the concentration of 1%” before using it in the mouth.5 This dilution is important because higher concentrations of hydrogen peroxide can easily damage intraoral tissue and cause gastric and colon disturbances. Proper concentrations of these mouthwashes are important to ensure that they are effective, yet not too strong to cause damage.9 However, a recent article studying the efficacy of hydrogen peroxide as a mouthrinse noted that at a concentration of 1%, hydrogen peroxide did not reduce the viral load of SARS-CoV-2 in intraorally positive subjects, and that rinsing creates a “false sense of security” in providers, so it should be cautioned against.10
The use of hydrogen peroxide as a disinfectant has been around for quite some time, but at concentrations much higher than acceptable in the mouth.11 Finding a safe yet effective concentration will be important to incorporate hydrogen peroxide into a mouthwash. According to recent studies, the SARS-CoV-2 virus persists on mucous membranes for two days before spreading to the lower respiratory tract.12 For this reason, it is proposed that rinsing with a form of hydrogen peroxide during this delay could prevent the virus from spreading to the lower respiratory tract, where it has the most negative effects.
Although hydrogen peroxide has not been explicitly studied for use as a mouthwash, studies of hydrogen peroxide 0.5% have been shown to inactivate SARS on inanimate surfaces in just one minute.13 Alternatively, another study of hydrogen peroxide in vitro concluded that the necessary low “clinically recommended concentrations of 3% and 1.5% had minimal virucidal activity after 15 seconds.”10 It is clear that compared to other studied mouthrinses, hydrogen peroxide has a lower efficacy in decreasing the viral load of SARS-CoV-2.
A similar mouthwash study looking at popular mouthwash brands and their effect on the SARS-CoV-2 virus categorized and studied mouthrinses based on their active ingredients. The three products containing hydrogen peroxide in this study “demonstrated similar abilities to inactivate HCoV” with an efficacy of “less than 90% to 99%,” which was markedly lower than mouthwashes containing a different active ingredient.13 More research should be performed on hydrogen peroxide with concentrations of 1.5%–3% as an antiseptic agent for oral and nasal rinsing at the onset of COVID-19 symptoms or as a preprocedural safeguard.
Povidone-iodine (PVP-I)
Povidone-iodine (PVP-I) is an antimicrobial agent effective against both gram-positive and gram-negative bacteria, as well as a large number of enveloped and nonenveloped viruses.14 Due to its efficacy in targeting other viruses, the ADA, in addition to recommending hydrogen peroxide, has also suggested gargling with 0.2% PVP-I before procedures to reduce COVID-19 transmission.9 Povidone-iodine works by oxidizing both amino acids and nucleic acids in its target.6 PVP-I can also be used to disinfect a wide variety of surfaces, which include the skin and mucosa. A series of studies have demonstrated PVP-I as having a “higher virucidal activity than other commonly used antiseptic agents,” which included chlorhexidine and benzalkonium chloride.15
A recent in vivo study on the effectiveness of PVP-I on the salivary viral load of SARS-CoV-2 had participants rinse with 15 mL of 1% PVP-I for one minute.16 The results of this test showed a significant drop in the viral load for half of the patients that lasted up to three hours. This was one of the first in vivo tests of its kind against the SARS-CoV-2 virus, and preliminary results show that more testing should be done with different concentrations of PVP-I to confirm it as a possible agent for mouthrinse in patients showing symptoms of COVID-19.
Another study confirms these findings using PVP-I diluted 1:30 with water to a concentration of 0.23% and gargled for 15 seconds, which resulted in viral titers being reduced by between 4.40 and 6.00 log10 TCID50/mL.14 PVP-I at a concentration of 0.23% seems to be “equivalent to 70% ethanol in inactivating” the COVID-19 virus in vitro.4
Additionally, a study comparing PVP-I and hydrogen peroxide found that PVP-I at concentrations between 0.5% and 1.5% with a 15-second contact time showed “complete inactivation” of the SARS-CoV-2 virus.9 This same study prepared PVP-I solutions at concentrations of 3.0%, 2.5%, and 1.0% with a 15-second contact time and observed the viral load decrease a value of more than 4.33 log10 CCID50.10 However, an alternate study suggests that 0.23% PVP-I is the lowest possible concentration still effective and shows that when 1% PVP-I is gargled for 30 seconds, the viral load is reduced by more than 99.99%.2
PVP-I has been active in vitro against other SARS and MERS coronaviruses, and thus should be researched for use as a prerequisite for nose, throat, or dental-related visits. In terms of sustaining a decreased viral load, a randomized control trial out of Singapore observed that the decreased salivary viral load was “sustained at three-hour and six-hour time points” when using povidone-iodine.17
Although PVP-I has shown great promise against SARS-CoV-2, it does have limitations given that it is contraindicated in people with hyperthyroidism, renal insufficiency, anaphylactic allergy, pregnant women, and people taking lithium-based antidepressants or undergoing radioactive iodine therapy.5,10 The other drawback of PVP-I is that current guidelines suggest not using it concurrently with other throat disinfectants, which could be inhibiting given that researchers are looking into how to combine these agents in a series of mouth and nasopharynx rinses to more significantly reduce the SARS-CoV-2 viral load.
Chlorhexidine
Chlorhexidine as an antiseptic has been proven effective against common enveloped viruses in vitro.3 Since SARS-CoV-2 is an enveloped virus, researchers have been looking into how to incorporate chlorhexidine into a mouthwash for patients recently diagnosed with COVID-19. A study conducted at the Korea University College of Medicine looked at the viral loads of SARS-CoV-2 in two patients with COVID-19 from days one through nine of their hospital stay.18 On days three and six, the patients rinsed with 15 mL of a 0.12% chlorhexidine gluconate mouthwash for 30 seconds. The viral loads were measured using rRT-PCR, and researchers found that the viral load in the saliva was significantly reduced for two hours after using the mouthwash, but it increased again thereafter.19 Although these results show promise, it should be noted that the sample size in this study is quite small and thus cannot be fully representative of the efficacy of chlorhexidine as a mouthrinse.
Furthermore, an in vitro study looking at the efficacy of oral rinses against the viral lipid envelope of SARS-CoV-2 using a 0.02% chlorhexidine rinse for 10 minutes found that it “only weakly inactivated coronavirus strains.”4 However, the same study suggested that chlorhexidine may be more effective in vivo where it can bind to oral surfaces and be released over time.4 Still other research suggests that chlorhexidine shows a “noneffective effect.”19
Alternatively, a 0.12% solution of chlorhexidine rinsed for 30 seconds has been shown to reduce the viral load transiently for two hours, but the viral load increases once again thereafter.18 When compared to povidone-iodine, a chlorhexidine mouthrinse did not significantly reduce the viral load and was found to have a varied efficacy against SARS-CoV-2, with a trend toward only weakly inactivating coronavirus strains.17 Another study investigated the effect of a series of mouthwashes on the SARS-CoV-2 virus and found that chlorhexidine can actually improve the symptoms of patients with COVID-19. More specifically, the use of a chlorhexidine mouthwash in critically ill patients was seen to reduce “ventilator-associated pneumonia from 24% to about 18%.”6 Although this study shows promising results, it will be necessary to perform more studies on chlorhexidine mouthwash with larger sample sizes to be able to make broader conclusions about its efficacy as a virucidal agent.
Other agents
Many of the initial compounds looked at are ones that have been useful in previous SARS and MERS viruses; however, there is a possibility that different chemicals from other sources may be effective as well, so the research should not remain too limited.20 For instance, a formula of Johnson’s Baby Shampoo diluted to 1% has been shown to be safe and effective in the treatment of rhinosinusitis.13 When studied against HCoV-229e, which was used as a surrogate for SARS-CoV-2 due to their similar pathogenicity, the 1% Johnson’s Baby Shampoo inactivated the virus more than 99.9% with a contact time of two minutes.13
Cetylpyridinium chloride is another compound found in many mouthwashes that destroys viruses by targeting the capsid or via lysosomotropic action and may be very effective against the SARS-CoV-2 virus.21
Lastly, mouthwashes containing chlorine dioxide, such as the brand OraCare, may be able to reduce the virucidal load of SARS-CoV-2 quite significantly as it has been proven effective against similar viruses. Chlorine dioxide works by oxidizing viral capsids by reacting specifically with cysteine, tyrosine, and tryptophan amino acid residues on the viral surface. It is a promising agent against SARS-CoV-2 because the spike protein of the virus contains high amounts of these key amino acid residues.22 Additionally, when tested against similar SARS viruses in wastewater, chlorine dioxide was shown to inactivate “94.4% of the virus after exposure of one minute.”23
More studies specific to SARS-CoV-2 will be necessary to confirm Johnson’s Baby Shampoo, cetylpyridinium chloride, and chlorine dioxide as virucidal competitors, but the idea of not limiting the scope of research remains. That being said, these chemicals hold great promise as virucidal agents, but further studies will need to be done to confirm proper rinsing times, concentrations, and locations for the agents to be most successful.
Discussion
Given the recent rapid rise of the SARS-CoV-2 virus, new research is currently being done on the mechanisms of the virus as well as possible agents that may be able to destroy it. Although much of this research is quite new, a review of the literature has shown that these three agents—hydrogen peroxide, povidone-iodine, and chlorhexidine—hold promise in the form of a mouthwash to reduce viral loads of the SARS-CoV-2 virus. The newer studied agents, such as Johnson’s Baby Shampoo and cetylpyridinium chloride, are also effective but must be studied further.
Much of the research already performed has focused on the efficacy of only one of these agents; therefore, it will be useful to compare agents in a controlled in vivo experiment using the same form of measurement and same conditions for each to see which one might hold the most promise against the SARS-CoV-2 virus. Additionally, many of these experiments contained small sample sizes, which may be due to the recency of the virus and the circumstances of the pandemic. Nevertheless, it will be necessary to increase these sample sizes for more accurate results that can be extrapolated to a larger population.
A study comparing all possible agents may lead to a further understanding of which chemicals may be safely combined to hypothesize a possible sequence of mouth and nasopharynx rinses that may reduce the viral load even more. This will require an understanding of these agents and their interactions as well as knowledge of limitations on surfaces in vitro. Most studies that have been performed have been done in vitro, but it will be important to take into account in vivo how the host immunity may impact the effect of the virucidal agent used, as this may change the efficacy.4 Likewise, putting these substances into the oral and nasal cavities may change the microbiota in those areas and could contribute to adverse effects; more research on these changes will be important before setting specific guidelines for mouthrinses.21
Conclusion
As previously mentioned, hydrogen peroxide, povidone-iodine, and chlorhexidine all hold great promise in efficiently reducing the viral load of the SARS-CoV-2 virus responsible for the COVID-19 pandemic. Since the virus is so new, much of the research surrounding these agents is not yet fully standardized as researchers find how to best measure the viral load in vitro and in vivo. For this reason, more research still needs to be done to confirm which agent is most useful and at what concentration and rinse time.
From our findings, it is clear that povidone-iodine reduces the viral load significantly and more consistently compared to other notable agents. However, it is important to keep in mind the limitations associated with studies of povidone-iodine, which include the lack of in vivo tests, small sample sizes, and possible contraindications of PVP-I in certain patients. Thus, it will be particularly important to confirm the proper concentration and rinse time for PVP-I such that it effectively reduces the viral load without damaging the intraoral tissue or causing harmful drug interactions. More in vivo tests are suggested to confirm the efficacy of each of these antiviral agents.
Related articles
- Lowering the transmission and spread of human coronavirus with over-the-counter products
- Molecular iodine as a new frontline defense against COVID-19 in the dental office
Editor’s note: This article first appeared in Through the Loupes newsletter, a publication of the Endeavor Business Media Dental Group. Read more articles at this link and subscribe here.
References
- Meister TL, Brüggemann Y, Todt D, et al. Virucidal efficacy of different oral rinses against severe acute respiratory syndrome coronavirus 2. J Infect Dis. 2020;222(8):1289-1202. doi:10.1093/infdis/jiaa471
- Khan MM, Parab SR, Paranjape M. Repurposing 0.5% povidone iodine solution in otorhinolaryngology practice in Covid 19 pandemic. Am J Otolaryngol. 2020;41(5):102618. doi:10.1016/j.amjoto.2020.102618
- Kelly N, Íomhair AN, McKenna G. Can oral rinses play a role in preventing transmission of Covid 19 infection? Evid Based Dent. 2020;21(2):42-43. doi:10.1038/s41432-020-0099-1
- O’Donnell VB, Thomas D, Stanton R, et al. Potential role of oral rinses targeting the viral lipid envelope in SARS-CoV-2 infection. Function (Oxf). 2020;1(1):zqaa002. Published online: June 5, 2020. doi:10.1093/function/zqaa002
- Basso M, Bordini G, Bianchi F, et al. Preprocedural Mouthrinses and COVID-19 Transmission: narrative Literature Review and Clinical Recommendations. Published online: June 14, 2020. doi:10.13140/RG.2.2.20306.38086
- Moosavi MS, Aminishakib P, Ansari M. Antiviral mouthwashes: possible benefit for COVID-19 with evidence-based approach. J Oral Microbiol. 2020;12(1):1794363. doi:10.1080/20002297.2020.1794363
- Naqvi AAT, Fatima K, Mohammad T, et al. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: structural genomics approach. Biochim Biophys Acta Mol Basis Dis. 2020;1866(10):165878. doi:10.1016/j.bbadis.2020.165878
- Tessema B, Frank S, Bidra A. SARS-CoV-2 viral inactivation using low dose povidone-iodine oral rinse—immediate application for the prosthodontic practice. J Prosthodont. 2020;29(6):459. doi:10.1111/jopr.13207
- Bidra AS, Pelletier JS, Westover JB, Frank S, Brown SM, Tessema B. Comparison of in vitro inactivation of SARS CoV‐2 with hydrogen peroxide and povidone‐iodine oral antiseptic rinses. J Prosthodont. 2020;29(7):599-603. doi:10.1111/jopr.13220
- Gottsauner MJ, Michaelides I, Schmidt B, et al. A prospective clinical pilot study on the effects of a hydrogen peroxide mouthrinse on the intraoral viral load of SARS-CoV-2. Clin Oral Investig. 2020;24(10):3707-3713. doi:10.1007/s00784-020-03549-1
- Ortega KL, Rech BdE, Costa ALF, Sayans MP, Braz-Silva PH. Is 0.5% hydrogen peroxide effective against SARS-CoV-2? Oral Dis. 2020. Published online: June 21, 2020. doi:10.1111/odi.13503
- Caruso AA, Prete AD, Lazzarino AI, Capaldi R, Grumetto L. Might hydrogen peroxide reduce the hospitalization rate and complications of SARS-CoV-2 infection? Infect Control Hosp Epidemiol. 2020:1-2. Published online: April 22, 2020. doi:10.1017/ice.2020.170
- Meyers C, Robison R, Milici J, et al. Lowering the transmission and spread of human coronavirus. J Med Virol. 2020;1-8. Published online: September 17, 2020. doi:10.1002/jmv.26514
- Eggers M, Koburger-Janssen T, Eickmann M, Zorn J. In vitro bactericidal and virucidal efficacy of povidone-iodine gargle/mouthwash against respiratory and oral tract pathogens. Infect Dis Ther. 2018;7(2):249-259. doi:10.1007/s40121-018-0200-7
- Vergara-Buenaventura A, Castro-Ruiz C. Use of mouthwashes against COVID-19 in dentistry. Br J Oral Maxillofac Surg. 2020;58(8):924-927. doi:10.1016/j.bjoms.2020.08.016
- Lamas LM, Dios PD, Rodríguez MTP, et al. Is povidone iodine mouthwash effective against SARS‐CoV‐2? First in vivo tests. Oral Dis. Published online: July 2, 2020. doi:10.1111/odi.13526
- Seneviratne CJ, Balan P, Ko KKK, et al. Efficacy of commercial mouth-rinses on SARS-CoV-2 viral load in saliva: randomized control trial in Singapore. Infection. 2020;1-7. Published online: December 14, 2020. doi:10.1007/s15010-020-01563-9
- Yoon JG, Yoon J, Song JY, et al. Clinical significance of a high SARS-CoV-2 viral load in the saliva. J Korean Med Sci. 2020;35(20):e195. doi:10.3346/jkms.2020.35.e195
- Farzan A, Firoozi P. Common mouthwashes for pre-procedural rinsing in dental practice: wich one is appropriate for eliminating coronaviruses? A mini literature review. Journal of Regeneration, Reconstruction & Restoration. 2020;5:e2. doi:10.22037/rrr.v5i1.29543
- Baker N, Williams AJ, Tropsha A, Ekins S. Repurposing quaternary ammonium compounds as potential treatments for COVID-19. Pharm Res. 2020;37(6):104. doi:10.1007/s11095-020-02842-8
- Burton MJ, Clarkson JE, Goulao B, et al. Antimicrobial mouthwashes (gargling) and nasal sprays administered to patients with suspected or confirmed COVID-19 infection to improve patient outcomes and to protect healthcare workers treating them. Cochrane Database Syst Rev. 2020;9:CD013627. Published online: September 16, 2020. doi:10.1002/14651858.CD013627.pub2
- Kály-Kullai K, Wittmann M, Noszticzius Z, Rosivall L. Can chlorine dioxide prevent the spreading of coronavirus or other viral infections? Medical hypotheses. Physiol Int. 2020;107(1):1-11. doi:10.1556/2060.2020.00015
- Karnik-Henry MS. Acidified sodium chlorite solution: a potential prophylaxis to mitigate impact of multiple exposures to COVID-19 in frontline health-care providers. Hosp Pract. 2020;48(4):165-168. doi:10.1080/21548331.2020.1778908
Amit Sachdeo, BDS, DMSc, MS, is an associate professor in the department of prosthodontics at Tufts University School of Dental Medicine. Contact him at [email protected].
Peter Arsenault, DMD, MS, MBA, is professor and head of the operative division and a professor in the department of comprehensive care at Tufts University School of Dental Medicine. Contact him at [email protected].
About the Author
Alexis Kaihlanen, MS
Alexis Kaihlanen, MS, is a second-year dental student at Tufts University School of Dental Medicine. Contact her at [email protected].
Amit Sachdeo, BDS, DMSc, MS
Amit Sachdeo, BDS, DMSc, MS, is an associate professor in the department of prosthodontics at Tufts University School of Dental Medicine. Contact him at [email protected].
Peter Arsenault, DMD, MS, MBA
Peter Arsenault, DMD, MS, MBA, is professor and head of the operative division and a professor in the department of comprehensive care at Tufts University School of Dental Medicine. Contact him at [email protected].