Battling biofilms
By J.W. Costerton, Ph.D., and Philip S. Stewart, Ph.D.
Defeating the toughest dental diseases begins with recognizing that bacteria aren't loners; they often live in complex communities called biofilms.
Pentagon planners concern themselves a great deal these days with information warfare. Why? Interfering with a foe's ability to communicate can be far more effective than destroying its bunkers or factories. In the battle against harmful bacteria, some investigators are considering the same strategy.
The microbes that cause many stubborn infections organize themselves into complex and tenacious films — biofilms — that can be nearly impossible to eradicate with conventional antibiotics. In the past few years, medical researchers have discovered that the microorganisms in biofilms depend critically on their ability to signal one another. Drugs able to interfere with this transmission might then bar the microbes from establishing infections or undermine their well-fortified positions; such agents might be used to treat biofilm infections like periodontitis, and might also be used to control the biofilms that form in dental unit water lines and threaten the pulmonary health of dental professionals and patients.
Signal-dampening compounds are currently being evaluated in animal studies, but why is it that such elegant weapons are only now being readied to enter the health-care arsenal? The answer, in short, is that microbiologists were training their microscopes on cultured cells suspended in a fluid droplet. That procedure is convenient but not entirely appropriate, because these experimental conditions do not reflect actual microbial environments. As a result, the bacteria in typical laboratory cultures act nothing like the ones encountered in nature.
In recent years, we and other bacteriologists have gained important insights into how common disease-causing microbes actually live. Our work shows that many of these organisms do not, in fact, spend much time wafting about as isolated cells. Rather, they adhere to various wetted surfaces in organized colonies that form amazingly diverse communities. In retrospect, it is astonishing that investigators could overlook this microbial lifestyle for so long. After all, bacterial biofilms are ubiquitous — dental plaque (which most of us confront daily), the slippery coating on a rock in a stream and the slime that inevitably materializes inside a flower vase after two or three days are but a few common examples. And bacteria, the focus of our studies, are not alone in the ability to create biofilms. Indeed, the genetic diversity of the microorganisms that can arrange themselves into living veneers and the breadth of environments they invade convince us that this ability must truly be an ancient strategy for microbial growth. Scientific appreciation and understanding of that strategy is, however, a modern phenomenon.
Germs in Flatland
The emergence of confocal scanning laser microscopy (CSLM) about 12 years ago enabled investigators to view slices of living biofilm at different depths and to stack these planes together to create a three-dimensional representation. Applying this approach in a concerted effort to study the structure of biofilms, John R. Lawrence of the Canadian National Water Research Institute, Douglas E. Caldwell of the University of Saskatchewan, and one of us (J.W.C.) demonstrated for the first time in 1991 that the bacteria grow in tiny enclaves, which we called microcolonies. Bacteria themselves generally constitute less than a third of what is there. The rest is a gooey substance the cells secrete, which invariably absorbs water and traps small particles.
The goo — or, more formally, the extracellular matrix — holds each microcolony together. A biofilm is built of countless such groupings, separated by a network of open water channels. The fluid coursing through these tiny conduits bathes each congregation of microbes, providing dissolved nutrients and removing waste products. The cells situated on the outside of a microcolony are well served by this plumbing system, but those in the interior are largely isolated. Because the goo mostly consists of water, small molecules can move through it freely, albeit with certain important exceptions. A substance will have a hard time diffusing to the center of a microcolony if it reacts with the cells or matrix material it encounters along the way.
Such chemical reactivity gives rise to small-scale environmental changes within a biofilm. These variations were recognized even before confocal microscopy revealed the cause. In 1985, our colleague Zbigniew Lewandowski began making direct measurements of chemical conditions in biofilms using needle-shaped microelectrodes with tips just one hundredth of a millimeter across. He found, among other things, that the oxygen concentration varies radically between locations as close as five hundredths of a millimeter apart — little more than the width of a human hair. Scientists often look at the amount of oxygen in a bacterial community because it can reflect the physiological status of the cells. For example, in a biofilm composed solely of Pseudomonas aeruginosa (the bacterium responsible for cystic fibrosis pneumonia), cellular activity and growth take place only where oxygen can penetrate — the outer two or three hundredths of a millimeter of each tiny colony. Deeper down, the cells are alive but dormant.
The variety of chemical environments that arise within a single biofilm means that one cell may look and act very different from the next even when the two are genetically identical. Similarly, local conditions control the production of many toxins and other disease-causing substances by microbial cells in a biofilm; consequently, some cells may inflict little harm on a host, whereas others may be lethal. The wide range of conditions can also permit several bacterial species to live side by side and thrive. It is fascinating that periodontitis appears to be "caused" by a consortium of at least six bacterial species, not one of which can be linked to the disease in all cases.
In certain cases, biofilms contribute to the health of their host. An example is the biofilms that form on fodder after cows or other ruminants eat it; these biofilms aid in the digestive process and also become the biomass that provides nutrition to the cow. For ruminant animals, these films are clearly indispensable. However, for the rest of us they are a nuisance or sometimes a serious threat to health. They can survive most chemical treatments used to control bacteria in healthcare and industry — treatments that would quickly eradicate free-floating cells. They can also evade the molecules and cells that the immune system unleashes. Biofilm infections thus tend to be quite persistent.
Tough bugs
Why, exactly, are these biofilms so resilient? At times, antibiotics and germicidal cleansers may fail to pierce the film. Penicillin antibiotics, for instance, have great difficulty penetrating biofilms containing cells that produce enzymes known as beta-lactamases. These enzymes degrade the antibiotic faster than it can diffuse inward, so that it never reaches the deeper layers of a biofilm.
Other factors enhance tenacity as well. Even when an antimicrobial agent penetrates biofilms easily, the microorganisms often still survive aggressive treatment that would eradicate free-floating cells. This ability had long mystified biologists, but lately they have learned that the variety of conditions and bacterial types in a biofilm confers protection against antibacterial agents. This paradox is especially apparent in periodontitis, in which biofilms occupy the deepening gingival crevices around affected teeth, forming macroscopic calcified masses that can be mechanically removed by root-planing operations. These massive biofilms are very accessible by direct entry into the infected sulcus or via the blood-derived gingival fluid, but periodontists still experience mixed results even with very aggressive antibiotic therapy.
Penicillin, for example, attacks replicating bacterial cells of many species. If a biofilm contains regions that are, say, starved of an essential nutrient, then the cells in those areas that are alive but not replicating will survive exposure to penicillin. Because active and inactive microbes are closely juxtaposed in a biofilm, and because surviving bacteria can use dead ones as nutrients, the few cells remaining after the antibiotic therapy ends can restore the biofilm to its original state in a matter of hours.
Such abilities explain why antimicrobials that work well on cultured cells often do not yield results that are useful to people doing battle with biofilms. Most of these people are health-care providers and patients, but a large number are engineers who have to contend with the ruinous effects of biofilms in industry, where bacteria often foul machinery and speed the corrosion of metal pipes. To aid both groups, in 1990 the National Science Foundation established the Engineering Research Center (now called the Center for Biofilm Engineering) at Montana State University, Bozeman, where the two of us have collaborated for nearly a decade.
Research here has revealed, among other things, that as bacteria adhere to a surface and form a biofilm, they manufacture hundreds of proteins not found in free-floating cells. Some of these proteins are involved in a curious shuffle that the cells carry out just after they settle on a surface but before they fix their positions, as Roberto Kolter and his colleagues at Harvard Medical School have shown by deleting certain genes (the blueprints for proteins) from various bacteria. Using Staphylococcus epidermidis, which is responsible for common staph infections, other researchers have identified genes that govern the next step in the development of a biofilm: the synthesis of the extracellular matrix. With these genes inactivated, the bacterium loses its ability to form a biofilm in the test tube and, apparently, in the tissues of laboratory animals.
How is it that the cells coming together to form a biofilm know to turn on certain genes in the first place? The answer is that these seemingly simple, autonomous microbes regularly communicate with one another. In P. aeruginosa and a broad class of similar bacteria, the relevant signaling molecules are acylated homoserine lactones, which each cell produces at a low level. When enough cells assemble, the concentration of these compounds increases, which in turn triggers changes in the activity of dozens of genes. David G. Davies of Binghamton University in Binghamton, N.Y. has shown that this mechanism, called quorum sensing, is critical for the development of biofilms. Indeed, laboratory strains of P. aeruginosa that lack the gene for a particular acylated homoserine lactone fail to build normal biofilms and instead pile up in a disorganized heap.
Now that biologists understand how bacterial biofilms form, controlling them with drugs able to target their unique properties should be possible. One might target the molecules that biofilm bacteria use to communicate, thereby halting biofilm formation or suppressing toxin production or other equally invidious activities. Instead of trying to overwhelm the offending organisms with poisons (and accidentally killing many more harmless or beneficial bacteria in the process), scientists will soon be able to manipulate the cells in more subtle ways to block their damaging activity.
Tactical warfare
Indeed, commercial development of at least one novel drug has already begun. Staffan Kjelleberg and Peter Steinberg of the University of New South Wales in Sydney, Australia, noted in 1995 that the fronds of a red alga (Delisea pulchra) growing in Botany Bay are rarely covered with biofilms. How do they do it? D. pulchra uses chemicals called substituted furanones to keep free of biofilms.
In the past few years, researchers have gained exciting insights into how the substituted furanones isolated by Kjelleberg and Steinberg work. These substances turn out to be similar to two classes of bacterial molecules: to the acylated homoserine lactones that many biofilm-making bacteria use for quorum sensing, and to a class of molecules, newly described by Bonnie L. Bassler of Princeton University, that virtually all bacteria emit to convey signals between different species. Apparently, the substituted furanones bind to bacterial cells at the sites normally used by the other signals and thus block the signaling molecules from delivering biofilm-promoting messages. Indications are that substituted furanones can both prevent biofilm formation and help to break up existing films. They also seem ideal for medical use because they are nontoxic and relatively stable in the body.
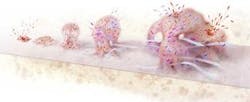
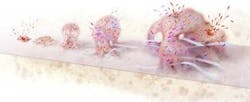
How biofilms form
- Free swimming bacterial cells alight on a surface, arrange themselves in clusters and attach.
- The collected cells begin producing a gooey matrix.
- The cells signal one another to multiply and form a microcolony.
- Chemical gradients arise and promote the coexistence of diverse species and metabolic states.
- Some cells return to their free-living form and escape, perhaps to form new biofilms.
null
J.W. ("Bill") Costerton, Ph.D., and Philip S. Stewart, Ph.D., have worked together for almost 10 years. Costerton, a bacteriologist, is head of the Center for Biofilm Engineering at Montana State University, Bozeman. Stewart, a chemical engineer, is deputy director and research coordinator at the center.