High-Tech Pulp Capping Using Laser and CAD/CAM
Article by Martha Cortes, DDS
The traditional pulp capping procedure is mechanical and uses chemicals to control bacteria, toxins, and bleeding. The most commonly used chemical is calcium hydroxide (CaOH2), reinforced by a layer of resin-modified glass ionomer cement since CaOH2 does not seal or adhere well. This is followed either by a temporary, composite, or amalgam restoration. In many cases, this mechanical procedure results in the need for a root canal on the same tooth.
During the past few years, I have had great success with a more high-tech approach to pulp capping. My procedure for treating deep carious lesions close to or touching the pulp uses a water-and-air-cooled erbium laser (Er,Cr:YSGG with a 2780 nm wavelength), Mineral Trioxide Aggregate (MTA) layered with a glass ionomer, and a CEREC computer-generated all-ceramic restoration, which is bonded using universal resin cement. This procedure, now my standard protocol, has significantly reduced the need for endodontic treatment.
This technique does not take too much time because it is part of placing a permanent, one-visit CEREC restoration. Prior to the procedure, I inform the patient of his/her tooth’s condition and the possible outcomes of different treatments. It’s no surprise that I receive virtually unanimous acceptance of a pulp capping technique that reduces or eliminates the need for a root canal.
Laser advantages
The mechanical removal of profound carious lesions leaves the pulp exposed to bacteria, which irritates it and leads to permanent damage and possible necrosis of the sensitive tissue.
Successful direct pulp capping requires the protection and preservation of the pulpal tissue beyond its actual state and condition. According to Blanken, instrumentation during excavation of a carious lesion is the most likely cause of pulp exposure. His prerequisite for successful treatment is the absence of bacterial contamination, and no thermal damage to the pulp.1
Unlike mechanical instrumentation, the erbium laser in this study produces minimal temperature increase because the tooth is air/water-cooled, while being bactericidal and productive of hemostasis.1-4 The laser also does not produce dentinal chips like rotary instruments, thus minimizing the chances of bacterial recontamination. These characteristics conform with Blanken’s prerequisites.
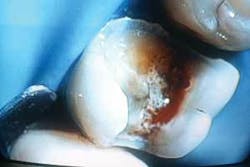
It should be noted that deep bacterial lesions are normally associated with large restorations that have experienced long-term microleakage or open margins, thus allowing bacterial infiltration at the pulp. These large restorations must be removed using an electric drill at low speed before a laser can be utilized (Figure 1). An electric drill produces less thermal damage at low speed than high speed, and is, therefore, less traumatic to the tooth structure.
null
The importance of biocompatible materials
Another consideration for success is the biocompatibility of the material used in direct pulp capping. A toxic pulp capping material will produce tertiary dentin due to excessive cytotoxic irritation; however, this dentin will attempt to protect itself from the irritant. These immune-disrupted dentinal bridges will be thin and incomplete, with empty spaces and tunnel defects.
Therefore, it is essential that the material be bio-friendly and alkaline in order to produce desirable results at the pulp/dentin interface.5,6 Bio-friendly materials such as MTA are only slightly reactive to the pulp, which produces the desired organized, restorative, and robust tertiary dentinal bridging.
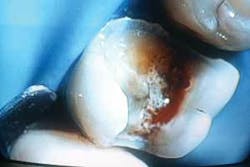
Once the lesion is ablated and disinfected with the laser, MTA is used as a liner to protect and alkalize the pulp tissue (Figure 3). MTA is also considered biologically friendly to tissue structure, yet is documented to produce tertiary dentinal bridge formation.5-8
MTA produces a restorative rather than a reactionary tertiary dentin, which is induced by a cytotoxic reaction to a nonbiocompatible pulp-capping material such as ZnOE (zinc oxide-eugenol). Similar to CaOH2, MTA produces a bacteriostatic and possibly bactericidal effect because it is alkaline. MTA is also gentler than CaOH2 to sensitive pulpal tissue, producing a milder but still effective response. This initiates tertiary dentinal bridging without reactive dentin getting in the way. This may be due to the fact that MTA contains calcium oxide rather than calcium hydroxide, and requires a longer drying time than CaOH2.
After the MTA is placed, it is covered by a glass ionomer cement to protect it while it dries and to protect the pulpal tissue. The resultant glass ionomer layer prevents microleakage to the pulp and creates a good interface with the dentinal tissue.9,10
Glass ionomers produce dentinal bridging, but are too irritating to be placed directly onto sensitive pulpal tissue. Chronic inflammation can also cause direct pulp cap failure, as the sensitive tissue is unable to deal with continuous inflammation. The glass ionomer is, therefore, seen as a secondary, yet essential step to successful pulp capping to prevent bacterial infiltration from compromised dentinal tubules.9 Studies confirm that the longer the pulp is exposed to bacterial trauma, the less likely it is to survive.1,9-12
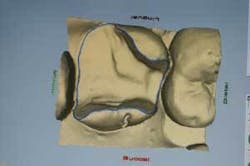
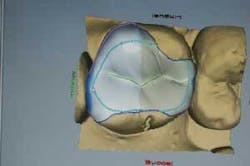
Following MTA and glass ionomer placement, a restoration must be placed as soon as possible to completely seal and protect the tooth from injury and bacterial invasion. Instead of placing a temporary and waiting up to two weeks for a lab-fabricated restoration, using the CEREC 3D System will enable the practitioner to design, mill, and place an inlay, onlay, or three-quarter crown in the same visit (Figures 4, 5, and 6).
null
null
null
Additional pulpal tissue protection with CEREC
The CEREC restoration is a conservative tooth preparation that minimizes loss of the essential enamel/dentinal tissue that protects the sensitive pulpal tissue, while minimizing microleakage or poor margins due to poor adhesion, which often occurs with metal or large porous full-ceramic crowns.
Since time is essential with this technique, I let the MTA dry under the permanent CEREC restoration. I believe this has a better biological effect, as the MTA is isolated and protected from the porcelain by a glass ionomer base (Fuji IX) that further seals the pulp tissue and protects the MTA from collapse or disintegration caused by the permanent restoration. The glass ionomer insulates the pulp from mechanical, thermal, or electrical trauma during and after treatment.
Finishing the restoration in one appointment increases success by sealing off the pulp from bacterial infiltration. Eliminating the need for a temporary also eliminates the need for its removal, which could introduce the pulp to bacteria and reinfection. According to Ritter and Swift, preserving the tooth structure and maintaining a bacteria-free interface in the restoration contribute equally to pulp protection.
The all-ceramic CEREC restoration is bonded to the tooth (Figure 7) using RelyX Unicem Universal Resin Cement (3M ESPE), a nontechnique-sensitive cement specially formulated to be self-adhering and moisture-tolerant. This eliminates the need for separate etching, priming, and bonding, thereby eliminating variables that could lead to a faulty seal.
Conclusion
It should be stressed that this technique is experimental. However, the vast majority of teeth treated to date remain asymptomatic, which anecdotally supports the technique and results.
Possible complications mentioned are eventual or immediate endodontic treatment and/or the obliteration of the pulp chamber due to chronic laser stimulation (iatrogenically produced) or infection. These possible scenarios should be considered and evaluated.
However, if endodontic treatment becomes necessary, the CEREC porcelain restoration will strengthen the tooth during treatment and help prevent fracture or disintegration of the coronal portion of the tooth. Use of a laboratory-fabricated ceramic (which would be necessary if there was no CEREC System) may compromise the pulp-capping technique, as it is partially based on sealing off the pulp from further trauma.
My clinical observations of this technique have been positive; however, the results described here would benefit from more long-term studies. Therefore, histopathological examinations would be helpful, possibly combined with scanning electron microscopy, to determine the extent of dentinal bridge formation, inflammation, cellular changes, and osteoblast differentiation. To obtain more statistically significant results, it would be necessary to increase the number of treatments and extend the observation period to 48 months.
References
- Blanken JW. Direct pulp capping using an Er,Cr:YSGG laser. J Oral Laser Applications 2005; 5:107-114.
- Rizoiu I, Kohanghadosh F, Kimmel A, Eversole LR. Pulpal thermal responses to an erbium, chromium: YSSG pulsed laser hydrokinetic system. Mosby, Inc.
- Eversole LR, Rizoiu I, Kimmel A. Pulpal response to cavity preparation by an erbium, chromium: YSGG Laser-powered hydrokinetic system. JADA 1997; 128:1099-1106.
- Glockner K, Rumpler J, Ebelseder K, Stadtler P. Intrapulpal temperature during preparation with the Er:YAG laser compared to the conventional bur: an in vitro study. J Clin Laser Med Surg 1998; 16.
- Queiroz A, Assed S, Leonardo MR, Nelson-Filho P, Silva LAB. MTA and calcium hydroxide for pulp capping. J Appl Oral Sci 2005; 126-30.
- Salako N, Joseph B, Ritwik P, Salonen J, John P, Junaid TA. Comparison of bioactive glass mineral trioxide aggregate, ferric sulfate, and formocresol as pulpotomy agents in rat molar. Dent Traumatol 2003; 19:314-320.
- Aeinehchi M, Eslami B, Ghanbariha M, Saffar AS. Mineral trioxide aggregate calcium hydroxide as pulp-capping agents in human teeth: a preliminary report. International Endodontic Journal 2003; 36:225-231.
- Holland R, Souza V, Nery MJ, Faraco I, Bernabe P, Otoboni J, Dezan E. Reaction of rat connective tissue to implanted dentin tube filled with mineral trioxide aggregate, portland cement or calcium hydroxide. Braz Dent J 2001; 12(1):3-8.
- Ritter AV, Swift EJ. Current restorative concepts of pulp protection. Endodontic Topics 2003; 5:41-48.
- Costa CAS, Oliveira MF, Giro EMA, Hebling J. Biocompatibility of resin-based materials used as pulp-capping agents. International Endodontic Journal 2003; 36:831-839.
- Tziafas D. The future role of a molecular approach to pulp-dentinal regeneration. Caries Res 2004; 38:314-320.
- Clinical Affairs Committee-Pulp Therapy Subcommittee. Guideline on Pulp Therapy for Primary and Young Permanent Teeth. Reference Manual 2004-2005.
Dr. Martha Cortes is a 1985 graduate from the University of New York at Buffalo School of Dental Medicine. She completed her residency training at Mount Sinai Medical Center in New York City, and has been affiliated with Mount Sinai as a faculty member since 1987. She is past president of the American Academy of Cosmetic Dentistry-New York Chapter, the past international chair serving consecutive terms, and has been an accredited member since 1992. She is past president of the Latin American Cultural Center, and was a reviewer of research grants for the Federal Medical agency. The United States Surgeon General, Dr. Antonia Novello, awarded her a certificate of achievement in 1993. She was the consecutive co-chair of dentistry with the American Society for Laser Medicine and Surgery for 2000-2001. Dr. Cortes is a qualified laser educator, an examiner for laser qualifications for the Academy of Laser Dentistry, and has a mastership in laser technology through the Academy of Laser Dentistry. She is a fellow of the international Academy of Facial-Dental Esthetics, a fellow of the American Society for Laser Medicine and Surgery, and a Diplomat Member of the American Society of Dental Aesthetics. Contact Dr. Cortes at [email protected].