Current concepts in dental bone grafting materials
Over the 100-year history of bone replacement in the human body for various purposes, a wide variety of surgical approaches and materials have been used.2 While autogenous bone from the patient remains the gold standard given all of the inherent growth factors, harvesting the bone may require a secondary surgical site, which is often undesirable from the patient’s perspective.
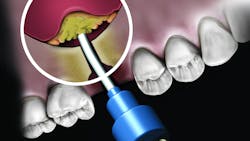
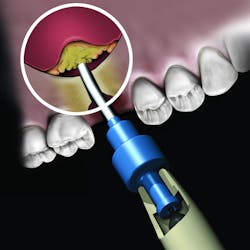
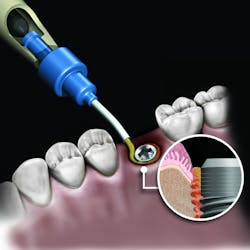
There are numerous products in each of the grafting categories that have evidence-based research supporting their efficacy. This has led some companies to look at alternative means of differentiating the product. One such example is the synthetic bone graft putty from NovaBone Products, LLC. The NovaBone Putty is a bioactive glass material with calcium phosphosilicate as the active ingredient (70% by volume). The material is radiopaque, has no risk of disease transmission given its synthetic origin, and has been shown to be osteostimulatory, meaning the graft helps accelerate bone formation in part due to the localized release of calcium and phosphate ions that play a role in the recruitment and formation of bone-forming cells (osteoblasts).3
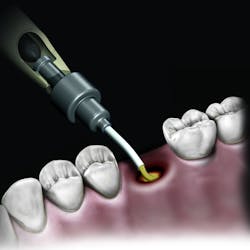
But the delivery system is what makes this product truly unique. While available in traditional syringe and clam-shell configurations, NovaBone Putty is the only bone graft material available in a cartridge configuration. Expressed with a dispenser, clinicians will be familiar with this delivery technique as it is similar toNovaBone products are exclusively distributed in the US and Canada by Osteogenics Biomedical.
Editor’s note: This article first appeared in Through the Loupes newsletter, a publication of the Endeavor Business Media Dental Group. Read more articles at this link and subscribe here.
References
- Cha HS, Kim JW, Hwang JH, Ahn KM. Frequency of bone graft in implant surgery. Maxillofac Plast Reconstr Surg. 2016;38(1):19. doi:10.1186/s40902-016-0064-2
- Horowitz RA, Leventis MD, Rohrer MD, Prasad HS. Bone grafting: history, rationale, and selection of materials and techniques. Compend Contin Educ Dent. 2014;35(4 Suppl):1-6.
- Profeta AC, Prucher GM. Bioactive-glass in periodontal surgery and implant dentistry. Dent Mater J. 2015;34(5):559-571. doi:10.4012/dmj.2014-233
Lou Shuman, DMD, CAGS, is the CEO of Cellerant Consulting Group, a leading dental corporate incubator and accelerator. He is a venturer-in-residence at Harvard’s i-Lab, the chairman of the technology advisory board at WEO Media, a member of the editorial advisory board of Dental Economics, and founder of the Cellerant Best of Class Technology Awards.
About the Author

Lou Shuman, DMD, CAGS
Lou Shuman, DMD, CAGS, is the CEO of Cellerant Consulting Group, a leading dental corporate incubator and accelerator. He is a venturer-in-residence at Harvard’s i-Lab, the chairman of the technology advisory board at WEO Media, a member of the editorial advisory board of Dental Economics, and founder of the Cellerant Best of Class Technology Awards.