QLF™-A new diagnostic tool for oral health assessment
Abstract —
Standard methods for caries detection by visual examination combined with tactile explorer response and radiographs are limited. Limitations include the inability to detect early bacterial activity within fissures, beneath the surface of the tooth or below the surfaces of restorations.
Quantitative light-induced fluorescence (QLF™) is a new method for oral health assessment, providing additional visual information about caries and bacterial activity. This new method uses the principle of fluorescence for visual enhancement of caries detection and bacterial activity. The method relies on contrast differences between sound and demineralized dentin by fluorescence. Specificity for caries detection and longitudinal changes in de/remineralization is enhanced over current methods.
QLF™ is on the verge of moving from research into clinical practice, after a long period of study and validation. This method has enhanced ability over standard visual methods to provide: assessment of caries, À assessment and quality improvement of sealants and restorations, and à visual aid for patient education in caries prevention. This paper describes several cases and future applications of QLF™ for clinical practice.
Introduction
QLF™ is a novel method for detecting caries and bacterial activity of teeth. A recent National Institutes of Health and National Institute of Dental and Craniofacial Research (NIH) report has noted that flipping a coin may provide as much sensitivity and specificity as current methods for diagnosing caries.1 Various papers have quantified the superior ability of QLF™ over standard methods to detect decay and bacterial activity in research settings.2,3,4,5,6,7 However, the application of QLF™ to clinical practice has not been well-documented. This paper discusses how this novel method of caries detection works and provides several clinical cases utilizing QLF™ for diagnosis. The last section discusses future applications and uses of QLF™ in dental practice, and summarizes research supporting its use for nonsurgical interventions.
QLF™ - How does it work?
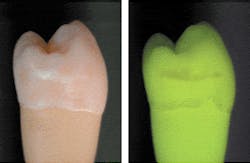
The basic principle of QLF™ is simple in that the principles of fluorescence are used to visually contrast sound and carious enamel and dentin. Basically, the observed natural fluorescence of a tooth is decreased due to increased scattering when a carious lesion is present.8,9 In 1978, Folke Sundstrom, a Swedish dentist at the Karolinska Institute in Huddinge, Sweden, discovered that teeth fluoresced with a distinct green light when illuminated with a specific spectrum of light in the violet-blue range (Figure 1).
Early lesions
The green fluorescing light is caused by the process of fluorescence. Certain elements in the tooth temporarily absorb the illuminating blue light and emit light with a green color. Folke also found that, when looking at the so-called auto-fluorescent image of teeth, early lesions turned up as dark, rather than white, spots. He also found that the contrast between the sound tooth-tissue and the lesions was much higher than when seen under normal white-light conditions.10,11 In-vivo research has shown that this green fluorescence permits visual detection of lesions invisible on radiographs and missed in standard visual examination.3,12 Using QLF™, there are approximately two- to tenfold more lesions detected vs. visual examination, visual tactile, or visual in combination with radiographs, depending on surface-type.3,12 Sensitivity values for QLF™ in comparison with histology ranged from 0.77 to 0.93, and specificity ranged from 0.70 to 0.75.13,14 In one study, the visual detection criteria resulted in sensitivity values too low to report, with only four lesions found.13 The other study reported a sensitivity of 0.83 and specificity of 0.66 for visual examination when Ekstrand criteria15 were used. With the inclusion of software with QLF™, fluorescence levels of sound and carious tooth-tissue were quantified16 with precise readings, allowing the technique to be named QLF™. This quantification allows detection of early lesions and follow-up of these lesions over time to determine whether further demineralization or remineralization is occurring in the teeth (Figure 2). With this method, the demineralization process that precedes every carious lesion can be followed over time. This is the oldest and most validated application of QLF™.2,17
QLF™ for detecting bacterial activity — an added value
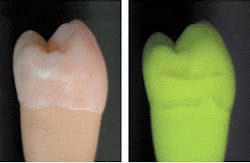
Beginning about 1995, when the first lamp-based clinical QLF™ instrument was introduced (as opposed to a laser system), a new phenomenon was observed. In some QLF™ images, areas on the teeth displayed a red hue that ranged from barely noticeable to very pronounced (Figure 3). This red light was also fluorescence, but not emanating from the teeth themselves. Research has shown the red fluorescence to be caused by certain byproducts of bacterial metabolism, mainly porphyrins.6 In general, this red fluorescence corresponds with mature plaque (older than three to five days), calculus, or gingivitis, independent of gross unremoved food particles. Often, the red fluorescence can be removed by professional cleaning. In some cases (Figure 3), no amount of nondestructive cleaning can remove the red fluorescence and the activity may be contained within the tooth enamel and dentin.
The green auto-fluorescence can show lesions at a very early stage. In contrast, white spots of demineralization that show red fluorescence are believed to have progressed to a state in which the enamel structure is so porous that bacterial meta-bolites, if not the bacteria themselves, can enter the lesion. Because the red fluorescence can be very strong (compared to the green auto-fluorescence), the fluorescence would raise the index of suspicion that caries is hidden underneath an otherwise sound surface. Very recently, digital image analysis that can enhance and objectify the amount of red fluorescence in a QLF™ image19 has become available.
Equipment
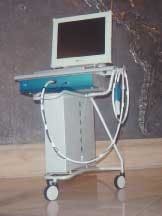
The only currently available clinical QLF™ system is the Inspektor Pro, shown in Figure 4. The system will be launched in Europe this fall. Availability in the United States is planned for early next year, pending FDA approval. The system is housed in a system box, with the handpiece connected to the system box through a cable that holds the light guide and video cable. The system box is mounted in a cart, which can be easily moved with a computer, medical flat-panel monitor, wireless keyboard, and a mouse. A footswitch is also available. Computer image, trolley, and system box are available as a complete package. Following initial installation, the system can be used without additional installation steps. The equipment is used like an intraoral camera allowing the operator to capture and store images of every tooth surface in the oral cavity.
The ALS ensures correct illumination of the teeth, excluding external (ambient) light, which could potentially interfere with the quality of QLF™ images. The ALS provides a sturdy support, aiding in repositioning of the camera for follow-up images. For infection control, the handpiece (Figure 4b) is covered with a disposable plastic cover and then fitted with a disposable cover called Ambient Light Shield (ALS) (Figure 4c). The ALS cover is available as a sterile disposable.
The software delivered with the caries-detection system allows easy imaging with automated name assignment of images linked to patient status over time. These longitudinal images can be viewed simultaneously or as a digital movie to show a patient changes over time. The system is equipped with an option for ungrading software as available.
Please note that the system allows quantification of the level of bacterial activity based on software analysis of the amount of red fluorescence accompanying any demineralization in the tooth. Following are two clinical cases using QLF™ technology.
Case 1 — Sealants
A problem faced by many practicing clinicians is assessment of the integrity of sealants. While sealants have been shown to be effective in preventing occlusal caries, detection of caries underneath sealants is difficult to determine with current visual examination.20 To date, QLF™ is the only method able to visualize and monitor caries under sealants.
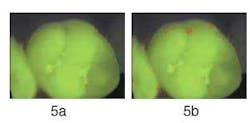
Figure 5b — Analysis for red fluorescence shows only very small areas with high red fluorescence. Red fluorescence analysis is visualized in pseudo-colors on top of the original image.
This enhanced method of visual examination with QLF™ allows clinicians to assess the current state of a sealant and, if necessary, monitor the sealant over time. Unlike other available visual techniques or radiographs, the area underneath the sealant can be monitored. Figures 5 and 6 show two examples.
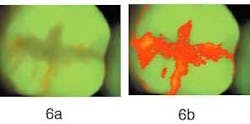
Figure 6b — The same sealant as the one on the left after RF analysis. The entire sealant and margins have a higher red fluorescence than the surrounding tooth tissue.
Figure 5a demonstrates a molar with an intact sealant, with little — if any — red fluorescence visible. This is confirmed when analyzing the image for the presence of red fluorescence as shown in Figure 5b; only a few tiny areas of red fluorescence on the surface were found. In Figure 6a, we see another molar with a sealant. Significant amounts of red fluorescence are viewed along the edges of the sealant and along the fissures. Since the molar was professionally cleaned prior to producing the image, the red fluorescence originates from under the sealant. In Figure 6b, the results of red fluorescence (RF) analysis are displayed in pseudo-colors to enhance the difference between areas with and without red fluorescence. RF analysis confirms that significant red fluorescence is emanating from under the sealant and in the fissures. While this method has not yet been validated for sealants, QLF™ is the most promising method available to detect faulty sealants at an early stage because of the ease and speed by which an image can be made.
Case 2 � Surgical intervention (excavation of decay)
Another practical clinical problem concerns excavation and how far to treat or when to stop the surgical intervention. Current methods of visual examination for caries with radiographs lack sufficient sensitivity and specificity to discriminate between sound and carious tissue. To date, caries-disclosing dye is the gold standard for caries detection, with too many false positives (low specificity). In recent studies, QLF™ has been shown to be much more reliable in determining the extent of caries excavation than use of caries-detector dye or based on visual inspection alone.7,21
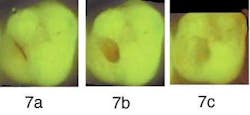
Figure 7a demonstrates a molar with a partly missing sealant, reported as sensitive for one month by a new patient aged 25 to 30, Visual inspection demonstrated a stained fissure and a partly missing sealant, without other remarkable signs.
Figure 7b — After surgically opening the fissure, the bright red fluorescence of the decay underneath is clearly visible.
Figure 7c — Following excavation, the preparation was completed because no additional red fluorescence is seen.
The initial QLF™ image (Figure 7a) of the molar demonstrated significant red fluorescence surrounding the stained fissure. Following initial surgical preparation, the carious lesion within the dentin demonstrated additional high-intensity red fluorescence (Figure 7b). The dentist proceeded with the excavation until she obtained Figure 7c with very little remaining red fluorescence. Research from Lennon7,21 et al suggests less affected dentin is removed than when using current visual methods for carious dentin removal, but validation studies are needed.
Future applications of QLF™
Perhaps some of the most important considerations beyond the lingering question of reimbursement for using this important new diagnostic method are decisions about the best options for prevention. The authors believe that QLF™ may have the greatest impact on nonsurgical preventive interventions and the ability to monitor the success of these. This is a promising area for use of the instrument and should be validated in additional research. QLF™ is unparalleled as a tool to evaluate the efficacy of remineralization on smooth surface lesions.17,22
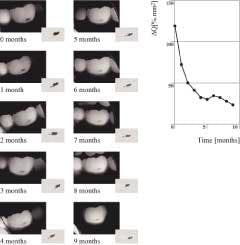
The QLF™ system can save and document the state of demineralization/remineralization of any tooth surface exclusive of proximal tooth surfaces at intervals in time. In addition, QLF™ can demonstrate disclosure of caries at a very early stage and assess the success or failure of a chosen prevention regime with objective, rather than subjective, criteria. Currently, the software quantifies only the amount of red fluorescence. The green fluorescence is not quantified, and changes can be viewed by viewing images simultaneously or visualized by showing the images as a movie. This has obvious advantages for use with patients to follow the development and progression of their own lesions on a screen.
For the first time, remineralization of white spots with fluoride treatment or other preventive measures can be documented over time. Because of the much higher sensitivity of QLF™ (ranging from 0.77 to 0.9313,14), evaluation of various nonsurgical treatment can occur over time. While visual examination can detect differences after six months, the above example demonstrates that QLF™ can detect significant differences and development trends in less time.2
A benefit using QLF™ may be in its ability to motivate patient behavior. Showing patients the bacterial activity with red fluorescence before-and-after pictures may be particularly useful in motivating patients in their self-care regimens. While additional behavioral research is needed to demonstrate the long-term benefits, showing patients the impact of their prescribed home-care regimens (e.g., proper brushing and use of home fluoride, etc.) may have a long-term benefit in adherence to recommended treatment of demineralized tooth surfaces.
Conclusions
QLF™ is a very promising addition to the dental practitioner's armamentarium. Many believe that it will become a standard of care for caries detection. Current systems are adapted for clinical use, ease of cleaning, and aesthetics within the office. Current software applications are evolving based on clinical application needs and the development of a scientific diagnostic nomenclature for caries. (e.g., International Caries Detection and Assessment System, (ICDAS)).23
Questions remain about the utility of the method for early caries detection without surgical interventions unless progress is made in reimbursement for this clinical service. Additional research is needed in this area, even though the disclosure and quantification of de- and remineralization is very well-documented and validated scientifically.
Red fluorescence of bacterial activity has much promise for the future, both for caries detection and patient education. The quantification is easy for bacterial activity and provides simple results. Validation studies are currently being conducted.
Perhaps the most burning question related to the early carious lesions remains: Now that an early carious lesion is identified, what course of action is recommended? There has been a trend in clinical practice to move toward minimum intervention dentistry, with atraumatic restorative technique (ART) and risk-based practices. For the moment, a nomenclature and corresponding treatment with surgical and nonsurgical interventions is emerging.23 As the new ICDAS nomenclature for caries evolves beyond the binary diagnostic system of caries or no caries, QLF™ may well be an essential tool for more sensitive staging of caries and treatment without surgical intervention. Reimbursement will be key to adoption of the technology.
Acknowledgements
We would like to acknowledge Drs. Roswitha Heinrich, Jan Künisch, and Petra Sas for providing clinical images.
For more information on QLF™ Technology, please contact OMNII Oral Pharmaceuticals at (800) 445-3386 or www.omniipharma.com.
References
- Pretty IA, Edgar WM, Higham SM, A review of the effectiveness of quantitative light-induced fluorescence (QLF™) to detect early caries, in Early Detection of Dental Caries III: Proceedings of the 6th Annual Indiana Conference, Stookey GK, Editor. 2003, Indiana University School of Dentistry: Indianapolis, Ind., USA; in print.
- van der Veen MH, de Josselin de Jong E. Application of quantitative light-induced fluorescence for assessing early caries lesions. Monogr Oral Sci 2000; 17:144-62.
- van der Veen MH, Buchalla W, de Josselin de Jong E. QLF™ technologies: Recent advances, in Early Detection of Dental Caries III: Proceedings of the 6th Annual Indiana Conference, Stookey GK, Editor. 2003, Indiana University School of Dentistry: Indianapolis, Ind., USA; in print.
- Simonsen RJ. Pit and fissure sealant: Review of the literature. Pediatr Dent 2002; 24(5):393-414.
- Lennon AM, Buchalla W, Switalski L, Stookey GK. Residual caries detection using visible fluorescence. Caries Res 2002; 36:315-319.
- ten Bosch JJ. Summary of research on quantitative light-induced fluorescence, in Early Detection of Dental Caries II: Proceedings of the 4th Annual Indiana Conference, Stookey GK, Editor. 2000, Indiana University School of Dentistry: Indianapolis, Ind., USA; 261-277.
- Pitts NB. Review of the ICW-CCT meeting — The importance of early detection and the philosophy and approach of ICDAS, in Early Detection of Dental Caries III: Proceedings of the 6th Annual Indiana Conference, Stookey GK, Editor. 2003, Indiana University School of Dentistry: Indianapolis, Ind., USA; in print.
Elbert Waller
Mr. Waller is co-founder and managing director of Inspektor Research Systems. He previously worked in the computer graphics section of the Technical University of Delft. Mr. Waller is currently preparing the market launch of QLF™. Contact Mr. Waller at [email protected].
Dr. Ir. Elbert de Josselin de Jong
Dr. de Jong is co-founder and technical director of Inspektor Research Systems. He studied physics at the University of Groningen and has a PhD in microradiography. He is responsible for R&D of diagnostic instruments caries research tools. Contact him at e.dejos [email protected].
Dr. Ir. Monique van der Veen
Dr. van der Veen is responsible for the development of QLF™ methodology at Inspektor Research Systems. She studied physics at the University of Groningen and did her PhD on the quantification of root caries lesions. Contact Dr. van der Veen at [email protected].