Dental Equipment and the Medical Model for Caries Diagnosis and Treatment
Story by Steven Steinberg, DDS | Photographer: Adira Edmund | Agency: Dreamstime.com
There’s a new paradigm for caries diagnosis and treatment. Our old surgical paradigm has served us well, but now it’s time to move on to a combined medical/surgical paradigm. We’ll call this the medical model. This article will review the medical model for the diagnosis and treatment of caries, as well as the role of dental equipment in this new model.
Caries diagnosis
Defining dental caries - We will define caries using the following two criteria. First, caries is a bacterial infection caused by specific bacteria in tooth biofilm. Second, caries is a multifactorial process of tooth demineralization and remineralization, which until cavitation is considered reversible.
Prognosis - In medicine, besides a diagnosis, there must be a prognosis. To prognose caries disease, its natural history and current epidemiology must be known. When does caries progress? When should we intervene with treatment? Should our treatment be surgical (fillings) or medical (antimicrobials or fluoride)? When we add a prognosis to our diagnosis, we end up with a far better treatment plan.
Two features provide a diagnosis/prognosis that leads to a treatment protocol. Caries activity describes the process (demineralization or remineralization) on an individual tooth surface. Caries risk describes the status of the whole patient, or the likelihood of the patient getting a new cavitation. Combining the risk and activity status gives us seven treatment groups, each with a combined medical-surgical antimicrobial treatment protocol based on this diagnosis/prognosis. We will now review the diagnosis of dental caries and the role of dental equipment.
Bacterial infection - The bacterial infection is due to Mutans Streptococci (MS) in the early stages and Lactobacilli (LB) in the later stages. Having both is a higher risk situation. MS can colonize in the mouths of predentate infants. Most children become infected by salivary exchange in the family unit between ages six months and 30 months. Early colonization by MS is a major risk factor for future caries.
It is important to note that caries is a bacterial infection, and that specific bacteria cause it. Loesche named this the specific plaque hypothesis, which states that it is not all plaque but specific plaque that contains odontopathogens that cause caries. Caries is now viewed as a specific infection of the entire mouth, not as a lesion on a specific tooth. Diagnosis is essential. Only patients at risk are treated, and the goal is to remove these organisms. Following primary treatment, patients are recalled to diagnose reinfection.
A test called CRT bacteria (Ivoclar Vivadent, Amherst, N.Y.) can diagnose the levels of MS and LB simultaneously. It is a selective media culture test that limits growth on a nutrient media to the target organism. The test can accurately diagnose the levels of bacteria in saliva, and has low sensitivity for caries (true positive) but relatively high specificity (true negatives). This means that the test is poor in predicting which individuals will get carious lesions based on specific salivary levels of the bacteria. However, the tests have high individual specificity, which means they are good at predicting low caries levels when the patient has a low bacterial count. Based on this information, microbial testing is reserved for continuing-care visits. It is used to monitor the return of organisms and check compliance with the therapeutics. This will be reviewed later.
A multifactorial reversible process - The development of dental caries is a dynamic process of demineralization that alternates with periods of remineralization. The bacteria in the plaque on the intact tooth surface metabolize sugar and produce acid. The acid penetrates the solid yet microscopically permeable tooth surface, driving calcium and phosphate out of the subsurface tissue and demineralizing it. The result is an initial white spot lesion.
Since the tooth surface is intact, the reverse biochemical process can occur. Saliva buffering can reverse the low pH in the plaque, and with the raised pH, calcium and phosphate can be driven back into the tooth to remineralize it. The key is the integrity of the tooth surface. If it remains intact (noncavitated), remineralization is possible. Then the question is, what is the prognosis? Is the lesion getting better or worse?
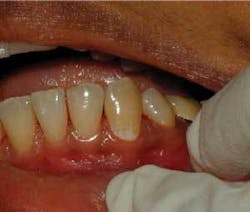
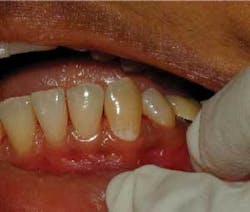
An active lesion is progressing toward cavitation (demineralizing), while an inactive lesion is not progressing or healing (remineralizing). If subsurface demineralization continues, it will eventually cause the collapse of the overlying tooth surface and create a cavitation. At this point surgical intervention - a filling - must be done. Until then, medical intervention is possible. The status of any noncavitated lesion is based on surface texture and color of the lesion. Using magnification under proper lighting and drying the tooth surface helps analyze the activity. White spot lesions may be considered active if they feel rough with an explorer and appear chalky, nonglossy, or matted (see Figure 1). This is due to its porous surface as it begins to have microporosities in the enamel. Inactive lesions have a relatively nonporous surface that feels smooth with an explorer and appears shiny.
Early diagnostic equipment - Sensitivity and specificity must be understood when assessing new instruments. Think of this example. Dentists would like their dental assistants to possess both speed and quality. While the two are often mutually exclusive, the goal is both. It is the same with sensitivity and specificity. The sensitivity of the device is a measure of how well it finds real disease, true positive findings. The specificity of the device is a measure of how well it finds real health, true negative findings. The higher the sensitivity, the lower the specificity tends to be. The higher the specificity, the lower the sensitivity tends to be. Sensitivity should be high with the medical/preventive paradigm (more early/benign/reversible treatments), and low with the surgical paradigm (fewer unnecessary, irreversible interventions).
Thus a new, more sensitive device can lead to overtreatment in the surgical model. If surgical intervention is only needed when there is a cavitation, devices with precavitation sensitivity might lead to precavitation surgical intervention (fillings) where medical treatment (remineralization) is indicated.
DIAGNOdent (KaVo America, Lake Zurich, Ill.) is a caries detection system based on laser-stimulated fluorescence. The DIAGNOdent is a battery-operated diagnostic device incorporating a solid-state diode laser that acts as an excitation light source and a photo diode that detects and measures fluorescence created by bacterial byproducts. A red diode laser with a wavelength of 655 nm emits light from a central optical fiber and directs the light into the tooth. It appears that fluorescence of the bacterial metabolites of the caries process exceeds that of healthy tissue. Surrounding the central laser fiber is a bundle of fibers that collect the reflected fluorescence from the tooth. This is passed through a high-pass filter and onto a photodiode that measures the amount of fluorescence. The degree of fluorescence is displayed as a number on the device (0 to 99).
In a recent systematic review, Bader and Shugars evaluated reports of the performance of DIAGNOdent. Detection of caries in dentin sensitivity and specificity values varied widely. When compared with visual assessment methods, the DIAGNOdent exhibited a sensitivity value that was almost always higher and a specificity value that was almost always lower. For the detection of occlusal enamel caries, the pattern that emerged from the in vitro studies is less definitive. There were too few comparisons between DIAGNOdent and visual methods. There was substantial heterogeneity (differences in methods and results) between most of the studies, thus precluding a meta-analysis of the performance data. There was also a problem in that the analyses used a variety of detection thresholds. Finally, DIAGNOdent does not tell the activity status of the carious lesions that are detected.
There are clinical implications from this review. The first is that we must be careful to use our new medical model with new diagnostic equipment. While DIAGNOdent offers increased sensitivity in detecting enamel in dental caries on occlusal surfaces, the improved sensitivity comes at the cost of specificity values that are lower than with visual examination. Remember, with our new paradigm, increased sensitivity and decreased specificity is OK. We will be doing reversible, medical procedures as opposed to surgery. The problem occurs if we keep our old paradigm and use DIAGNOdent scores alone as a guide to surgical invasion. We will be irreversibly treating some healthy teeth.
A second clinical implication is that we can use DIAGNOdent after visual examination to refine a questionable diagnosis in caries on the occlusal surface of the tooth. This could be very useful for hidden caries. Using a DIAGNOdent reading of greater than 30 can be helpful to verify dentinal caries. A third use for DIAGNOdent is to monitor lesion activity over time. This is not as well supported with today’s data as the other two uses. Further studies should clarify other uses of DIAGNOdent. Also of interest to clinicians is the new DIAGNOdent pen, which is a handheld portable caries detection aid. The manufacturer reports that it provides the same accuracy as the DIAGNOdent.
The Inspektor™ Pro preinvasive caries diagnostic imaging system uses quantitative light-induced fluorescence (QLF) technology. Teeth illuminated with the specific wavelength blue light from the handpiece emit light in the green part of the spectrum. The teeth appear to be lit from within as the fluorescence levels reflect the mineral content of the enamel. White-spot lesions appear dark, and bacterial endotoxins appear red. The QLF™ software makes it possible to capture in vivo images of the tooth in the computer. Live images are displayed in real time on the computer. Using an optimized capturing sequence, it takes only minutes to assess all tooth surfaces. A semiautomatic technique is used to quantify the lesion size, depth, and volume from the tooth images. The software is equipped with several tools to manage large databases of multiple patients.
The Inspektor™ Pro consists of a trolley that contains a computer and the Inspektor™ Pro System Box. The trolley makes the system easily transportable between operatories. The Inspektor™ Pro System Box contains the QLF™ light source and the hardware for the live video and patient isolation. Connected to the system box is the Inspektor™ Pro handpiece used to make the images in the oral cavity.
The software program allows users to capture virtually identical images of teeth time after time. A baseline image is taken to help detect and assess early caries. During subsequent visits, the handpiece is moved around the tooth surfaces in question. Based on predefined user settings, the computer can automatically capture and save a new image that most closely matches the baseline image. In this way, images at each visit are collected to compare over time. This tool helps find areas at risk before restorations are needed. Using the medical model, remineralization procedures can be implemented and tracked based on an analysis provided by the system. Progressive demineralization can also be tracked.
This technology is especially helpful in the research of dental caries. Researchers and dental manufacturers must test products with anti-caries claims for more than three years. With QLF technology, that time frame can be shortened considerably by quantifying the demineralization and remineralization over less time. Evaluation of new and existing products will become easier and less expensive.
In a search of PubMed, two studies compared DIAGNOdent with QLF. Aljahani et al. noted consistently better scoring with QLF compared to DIAGNOdent on extracted teeth with orthodontic appliances. It was concluded that QLF may be suitable for quantifying incipient carious lesions adjacent to fixed orthodontic appliances.
In the second article by Shi et al., it was noted that for lesion depth, correlation with the gold standard was similar for QLF and DIAGNOdent - about 0.85. With respect to caries detection, sensitivity for DIAGNOdent was 0.75 and specificity was 0.96, with a cut-off point of 9. The corresponding values for QLF were 0.94 and 1, with a cut-off value of 20 percent of fluorescence loss. Spearman’s rank correlation coefficients for enamel mineral loss for QLF and DIAGNOdent, respectively, were 0.76 and 0.67. It was concluded that for quantification of smooth-surface caries, the methods are of equal merit. But for scientific purposes, QLF offers the advantage of closer correlation with changes in mineral content.
A report by Tranaeus concluded that the QLF method is sensitive and suitable for longitudinal quantification of incipient caries lesions on smooth surfaces, and that repeated fluoride applications had a favorable effect on the remineralization of white-spot lesions measured after six months.
DIFOTI, or Digital Imaging Fiber-Optic Trans-Illumination (Electro-Optical Sciences, Irvington, N.Y.), was developed for early caries detection without ionizing radiation. DIFOTI uses fiber-optic transillumination of visible light to image the tooth. Light delivered by fiber optics is collected on the other side of the tooth by a mirror system and sent to a digital electronic charge-coupled device. The data are sent to a computer for analysis with dedicated algorithms, which produces digital images that can be viewed by the clinician and patient in real-time or stored for later use.
Young and Featherstone conducted a study to determine whether DIFOTI could evaluate early approximal lesions, and to compare radiographs produced with F-speed film with both histologic lesion depth and cavitation. The authors created artificial approximal lesions in vitro in extracted teeth over 14 weeks and imaged them using a “bitewing-like” view every two weeks with DIFOTI and F-speed radiographic film.
At the end of 14 weeks, the authors examined the lesions for surface cavitation using visual and tactile methods. They then thin-sectioned the lesions and subjected them to histologic analysis using polarized light microscopy (PLM). DIFOTI was not able to measure the depth of a lesion in any of the samples. It was, however, able to show surface changes associated with early demineralization as early as two weeks. The depth of a lesion measured using F-speed radiographic film was not statistically different from the depth of a lesion measured with PLM histologic analysis (P > .05). None of the lesions showed any signs of surface cavitation after 14 weeks of demineralization.
The authors concluded that DIFOTI technology should not be used to decide between surgical or chemical treatment strategies based on lesion depth. Although the DIFOTI manufacturer does not claim that the device measures lesion depth, many clinicians use it to make treatment decisions about whether to restore early proximal lesions. While DIFOTI images cannot determine the depth of a lesion, they can show signs of subsurface demineralization very early (two weeks). This reinforces the manufacturer’s view that DIFOTI is a good tool for detecting early surface changes and alerting clinicians to use clinical judgment and other detection methods. Ideally, the clinical decision whether to cut the tooth should be based on cavitation rather than histologic lesion depth.
Treating caries with a medical model
This early caries detection information enables more effective use of the medical model for caries treatment (Table 1). As discussed in a previous article (September/October 2004 Dental Equipment & Materials), by combining the three risk levels (Table 2) with the two states of caries activity, we formed seven treatment groups (Table 3). First, there is a low risk level, which will always be inactive (LR). The moderate risk level can be either active or inactive, depending on the presence or absence of chalky white spots. Finally, the high risk level has at least one tooth with a cavitation. However, other noncavitated teeth can have chalky white spots.
null
null
If there is only cavitation without chalky white spots on the other teeth, the patient is classified as high risk active (HRA). If there are cavitated teeth as well as other teeth with chalky white spots, this doubled activity is called high risk active active (HRAA). A special category, high risk inactive (HRI), denotes patients who have undergone treatment and are not active, but who require continued risk-reducing therapy. Patients who present with eight or more cavitated teeth are designated as very high risk (VHR).
Early detection of carious lesions at a demineralization stage rather than in the later cavitation stage enables the dental team to treat caries using remineralization strategies as opposed to restorative/surgical strategies. Remineralization strategies include the use of antimicrobial chemotherapeutics; i.e., fluoride varnish, chlorhexidine rinse, and xylitol gum. Strategies also include use of some form of calcium phosphate and fluoride at home.
Summary
This article reviewed the medical model for the diagnosis and treatment of caries as well as the role of dental equipment in this new model. Two key points are essential to understanding caries. First, caries is a bacterial infection caused by specific bacteria in tooth biofilm. Testing for bacteria was discussed. Second, caries is a multifactorial process of tooth demineralization and remineralization, which until cavitation is reversible.
We presented a discussion of early diagnostic equipment. Key to that discussion was the point that as our ability to detect caries at an early precavitated stage increases, we must take care to use a medical as opposed to a purely surgical approach to treatment. Precavitated demineralized carious lesions need to be remineralized rather than surgically restored; otherwise, significant overtreatment will ensue. Our paradigms must keep up with the advancements of science and technology.
Dr. Steven Steinberg has been in private practice since 1982. He has been the volunteer director of the Ark Dental Clinics in Chicago, and is currently a clinical consultant for the University of Illinois College of Dentistry continuing-education online course on dental caries. He has spoken at the Chicago Midwinter Meeting, the Hinman Dental Meeting, and around the world on the new paradigm in the diagnosis and treatment of dental caries. Dr. Steinberg is available for speaking and can be reached at [email protected].