Using an integrating porcine collagen membrane to decrease treatment times in complex implant cases
Guided bone and tissue regeneration procedures play an integral role in the daily practice of dental surgeons and an increasing number of general practitioners. By understanding the basic principles of wound healing, dental professionals can use biomaterials and surgical techniques to regenerate specific tissues lost due to disease, trauma, and the natural course of aging. In periodontal disease, the epithelial attachment, underlying bone, periodontal ligament, connective tissue, and cementum are all destroyed around the tooth (with the exception of the cementum and periodontal ligament around implants).
Guided tissue regeneration techniques, when performed properly, can predictably regenerate all of these tissues to aid in reestablishing a healthy and functional periodontal attachment apparatus around a tooth. Guided bone regeneration techniques use specific materials to create a space where bone can be regenerated in a specific compartment, in preparation for a dental implant, to support soft tissue at a pontic site, or for support of a removable prosthesis. Guided bone and tissue regeneration both require elevation of soft tissue and removal of any pathologic or causative agents and resultant reactive tissue for creation of a potential space where the desired tissue type(s) can form during healing.
The maintenance of space and the exclusion of undesired cell types are imperative for success and typically rely on a membrane, a graft material, or a combination of both.
Barrier membranes aid in graft containment, stabilization of the graft and blood clot, and exclusion of soft tissues. (1–3) Nonresorbable membranes are the “gold standard” but are not used as frequently as resorbable membranes due to high complication rates resulting from membrane exposure and infection. (4,5) Additionally, they require a second surgical procedure to remove. The optimal resorbable membrane causes minimal tissue reaction during and following the resorptive process. (6) Additionally, resorbable membranes have shown equal or greater efficacy than nonresorbable membranes in guided bone and tissue regeneration. (7,8)
Ossix Plus, manufactured by Datum Dental (Lod, Israel) and distributed in the United States by OraPharma (Bridgewater, New Jersey), is an integrating porcine collagen membrane indicated for use in guided tissue regeneration and guided bone regeneration procedures. "Integrating" refers to the fact that the membrane has been shown to histologically ossify or integrate into the tissue and underlying bone during the resorption process. (9,10) Typically, a resorbable collagen membrane must be present for a minimum of three months and preferably six months for most guided tissue regeneration and guided bone regeneration procedures. Collagen cross-linking allows the membrane's in situ life to be extended and improves handling properties. Cross-linking of collagen increases resistance to both tissue and bacterial collagenase-mediated breakdown of the membrane. The Ossix Plus membrane is cross-linked using patented Glymatrix technology, in which natural sugars (ribose) are used in the process of glycation to cross-link collagen molecules, rather than molecules such as glutaraldehyde. By using a naturally occurring process, a much smaller inflammatory response is elicited in vivo. As a result, the membrane and underlying graft and/or healing implant are, in most cases, not subject to excessive inflammation which can cause earlier disintegration of the membrane and subsequent regenerative materials. Ossix Plus has demonstrated consistent functional integrity for four to eight months, even with membrane exposure to the oral cavity where bacterial and tissue collagenase are present. (10–12)
Placement of dental implants into fresh extraction sockets (immediate placement) simultaneous with guided bone regeneration procedures in resorbed ridges results in dramatically reduced treatment times. With the use of appropriate surgical techniques and materials, it is possible to achieve predictable results with these procedures. To illustrate the attributes of a membrane with optimal barrier function, I will present two cases: one involving placement of a dental implant simultaneous with removal of a primary mandibular molar with guided bone regeneration, and one involving a ridge-split guided bone regeneration procedure simultaneous with implant placement.
Case No. 1: Extraction of primary tooth K and placement of immediate implant with guided bone regeneration
JC is a 57-year-old female with a retained primary tooth K and a congenitally missing No. 20. K was becoming increasingly mobile and developed bone loss beyond the apex of the distal root, necessitating removal of the tooth.
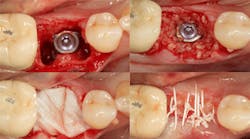
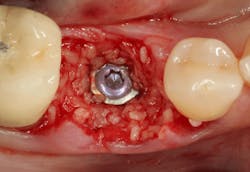
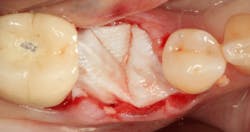
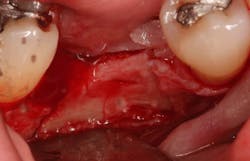
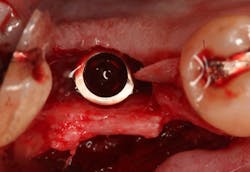
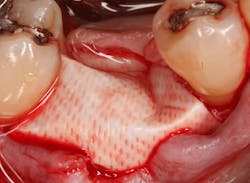
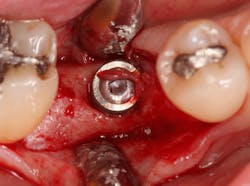
Excellent interseptal bone width was present, both buccal and lingual plates were present, and an adequate amount of bone was present coronal to the inferior alveolar nerve to allow for immediate implant placement. A sulcular incision was made around K and the adjacent teeth, and a full-thickness buccal and lingual flap was reflected. The tooth was sectioned in half, and the mesial and distal roots were elevated. The socket was degranulated with a round diamond bur and copiously irrigated with chlorhexidine gluconate 0.12% rinse. An osteotomy was completed in the interseptal area, and a bone-level 4.8- x 10-mm implant was placed to 35 Ncm. A mixture of cancellous and cortical allograft was infused with autogenous plasma-rich growth factor (PRGF), which was isolated during a preprocedural blood draw. The mixture was packed into the residual socket, slightly coronal to the implant platform to account for predictable resorption during healing. (13)
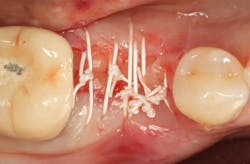
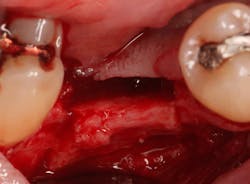
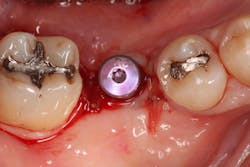
A 15- x 20-mm Ossix Plus membrane was hydrated in sterile saline for three minutes and trimmed to extend roughly 3 mm beyond the buccal and lingual extent of the bone graft and roughly 1 mm from the adjacent teeth. A sterile template is included in each membrane package; it can be trimmed to the appropriate dimensions and superimposed on the Ossix Plus membrane for final trimming to ensure the exact dimensions desired are obtained. This membrane can also be sutured, if necessary. When this technique is used, it is important to pass the suture needle through the membrane at a 90-degree angle to avoid tearing the membrane.
At this point, releasing incisions (vertical incisions and/or periosteal releasing incisions) can be made to achieve primary closure or to leave the membrane exposed and let the area heal by secondary intention. With the Ossix Plus membrane’s increased resistance to bacterial collagenase and the stresses of the oral environment, I feel comfortable leaving the membrane exposed and allowing the attached gingiva to heal over the membrane. This will result in reduced swelling and discomfort during healing, and there is less risk of damaging the mental nerve as it exits the mental foramen and fans laterally into the tissue. Attached gingiva around an implant will facilitate a more accurate impression, easier delivery of the restoration (and increased resistance to cement penetrating subgingivally), and easier hygiene for the patient. Also, when the implant is uncovered after healing, a tissue punch can be used, and there will generally be no need for a flap and sutures, thus improving the patient experience. Conversely, primary closure is imperative when performing a lateral or vertical ridge augmentation with or without simultaneous implant placement. Generally, when the extraction socket walls are intact, leaving the membrane exposed and allowing attached gingiva to grow over the membrane is predictable.
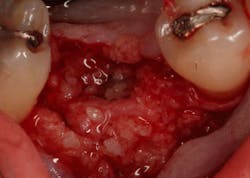
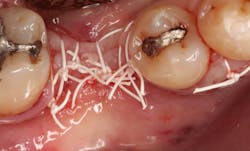
In this case, I placed an autogenous fibrin clot, which was isolated from the fractionated blood draw, over the Ossix Plus membrane and sutured, without obtaining primary closure, using a CV-5 Gore-Tex (W.L. Gore & Associates Inc.). This technique is designed to help facilitate the formation of a blood clot over the Ossix Plus membrane. It can be used with the addition of a collagen plug over the membrane, or you can just suture and allow a natural clot to form.
The patient was placed on Augmentin for 10 days, given anti-inflammatory pain medication (flurbiprofen, 100 mg, bid), and instructed to rinse with chlorhexidine gluconate 0.12% rinse for one minute in the morning and one minute prior to bedtime. The patient was instructed not to eat or drink anything for 20 minutes after rinsing with chlorhexidine gluconate 0.12%. The sutures were left in place for two weeks, and the patient was instructed not to brush the teeth adjacent to the No. 20 space during that time.
The patient returned for a two-week suture removal and was given an ultrasoft postsurgical brush. The patient continued chlorhexidine gluconate 0.12% rinses for one week and was instructed to brush the adjacent teeth and gums with the postsurgical brush for one week. The patient was then told to resume normal hygiene in the area and discontinue chlorhexidine gluconate 0.12% a week after suture removal (i.e., three weeks from surgery).
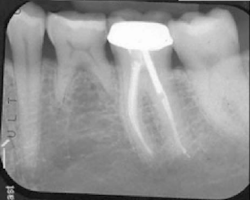
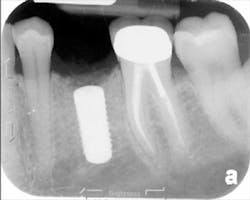
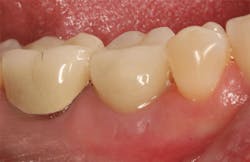
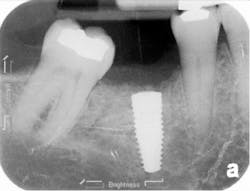
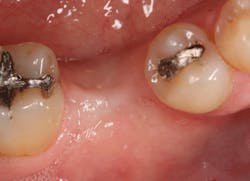
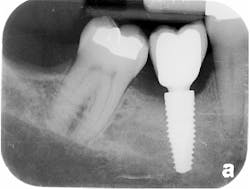
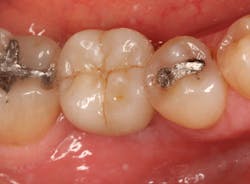
The patient was seen four months later for Stage II uncovering of her implant using a tissue punch and was sent to her restorative dentist for final impressions two weeks after that. Three months after the initial surgery, the patient returned with her final restoration in place for a soft-tissue exam and radiograph. Six months after surgery, she returned for a postdelivery radiograph, shown here. (I see every implant patient three months after seating the final restoration to check for residual cement, ensure that the restorative apparatus is fully seated, and check the soft- and hard-tissue response to the new restoration. This is an extremely important—and often overlooked visit—where problems are often noted that can cause significant damage to the bone and gingiva if early intervention is not initiated.) The bone levels were well-maintained around the implant platform, and the soft-tissue health was excellent. The patient reported a high degree of satisfaction with her new implant restoration.
Case No. 2: Ridge-split guided bone regeneration procedure and simultaneous implant placement
NC is a 68-year-old male who had been missing No. 30 for roughly 15 years. Horizontal and vertical ridge resorption was present at the edentulous site. Additionally, mesial tipping of No. 31 had occurred. Horizontal bone augmentation was required to place a standard-diameter implant in an ideal restorative location. A ridge-split technique was chosen to move the buccal plate in a more buccal direction and create a widened space in the ridge for ideal implant placement and to house an interpositional bone graft.
A crestal incision was made on the edentulous ridge and sulcular incisions were made on adjacent teeth. A partial-thickness flap was elevated on the facial to maintain a periosteal blood supply to the buccal plate. The periosteum was left on the buccal plate to provide additional blood supply by using a sharp dissection (whereas a blunt elevation [full-thickness flap] would have involved elevation of the periosteum off of the bone with the flap).
A Piezosurgery device with saw inserts was used to create strategic cuts at site No. 30 down the center of the ridge to a depth of 10 mm. Piezosurgery uses ultrasonic vibrations to remove bone, much like a high-speed handpiece but causing less inflammation. Oblique cuts in the buccal bone were made diverging apically to make the base of the isolated portion of the buccal plate wider than the coronal portion to allow for maximum blood supply. A shallow cut was made in the apical portion of the bone, connecting the vertical cuts to allow for the newly isolated trapezoidal piece of buccal plate off which to hinge. A mallet and chisels were used with gentle tapping to out-fracture the buccal bone and to create space in the middle of the ridge. Careful attention was made to ensure that the apical portion of the split ridge was kept intact to allow for blood flow to the isolated portion of bone.
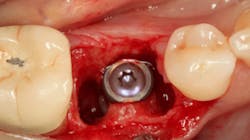
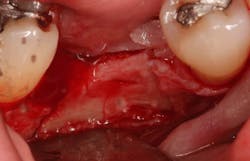
An osteotomy was then created, extending to the apical portion of the split ridge in preparation for implant placement. A bone-level tapered 4.1- x 12-mm implant was placed with a flat cover screw. A mixture of cancellous and cortical allograft particulate was infused with autogenous PRGF and packed around the implant on the mesial and distal aspects. The newly out-fractured buccal plate was positioned intimately adjacent to the buccal portion of the implant, and additional graft material was placed over the buccal plate to further augment the bone horizontally and reduce the risk of buccal-plate resorption during healing. A 25- x 30-mm Ossix Plus membrane was soaked in saline for three minutes and trimmed to extend 3–4 mm beyond the confines of the bone graft and roughly 1 mm from the adjacent teeth.
After the photo was taken to demonstrate the membrane placement, a periosteal elevator was used to further push the membrane under the lingual flap so that it draped intimately over the graft. The partial-thickness flap preparation made during initial incisions allowed for coronal positioning for tension-free primary closure. A CV-5 horizontal mattress suture was used to bring the buccal and lingual flaps together, and closure was completed with interrupted sutures.
The patient was placed on Augmentin (875 mg, bid) for 10 days and instructed to rinse with chlorhexidine gluconate 0.12% rinse for one minute in the morning and one minute prior to bedtime. Flurbiprofen and acetinominophen were used for pain management. The same timeframe for suture removal and the same postoperative hygiene instructions were recommended.
Stage II uncovering was performed five months following the initial surgery. A crestal incision was made toward the lingual ridge to split the keratinized gingiva, as that is where the keratinized gingiva had settled following surgery due to the coronal advancement of the buccal flap to obtain primary closure. The keratinized gingiva was buccally positioned, and a healing abutment was placed. The tissue was sutured with 4.0 chromic gut. This allowed the attached gingiva to heal on the buccal portion of the healing abutment and thus remain on the buccal portion of the final restoration. Final impressions were taken two weeks later, and the restoration was delivered thereafter.
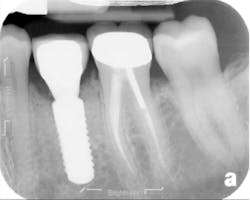
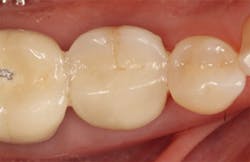
The patient returned three and six months later for a postdelivery radiograph and healing check. The peri-implant tissues were healthy with no abnormal bleeding or probings. The patient reported high satisfaction with his new implant restoration.
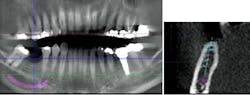
These challenging cases provide examples of how the overall treatment time and number of surgical interventions can be minimized with a well-planned and -executed guided bone regeneration procedure simultaneous with implant placement. This involves cone-beam computed tomography (CBCT) imaging for preoperative planning, selection of the appropriate surgical technique, and application of predictable materials where necessary.
A tremendous amount of regenerative materials are on the market today from a wide array of manufacturers, distributors, and tissue banks. When selecting a product, it is extremely important that the practitioner examines the scientific and clinical research demonstrating application of the product in the particular clinical context that it will be used. The Ossix Plus membrane is one of the most well-researched and documented membranes on the market today. The Ossix Plus membrane is supported by sound scientific evidence that shows consistent and predictable integration into the underlying bone and predictable resorption time, even with exposure to the oral cavity. For these reasons, it is my membrane of choice for my guided bone and tissue regeneration applications.
If you have any questions on any topic discussed in this article please contact me at [email protected].
References
1. Dahlin C, Linde J, Gottlow J, Nyman S. Healing of bone defects by guided tissue regeneration. Plast Reconstr Surg. 1988;81:672–676.
2. Hammerle CH, Karring T. Guided bone regeneration at oral implant sites. Periodontol. 2000;17:151–175.
3. Buser D, Bragger U, Lang NP, Nyman S. Regeneration and enlargement of the jaw bone using guided bone regeneration. Clin Oral Implants Res. 1990;1:22–32.
4. Hammerle CH, Jung RE. Bone augmentation by means of barrier membranes. Periodontol. 2000;33:36–53.
5. Schliephake H, Kracht D. Vertical ridge augmentation using polylactic membranes in conjunction with immediate implants in periodontally compromised extraction sites: an experimental study in dogs. Int J Oral Maxillofac Implants. 1997;12:325–334.
6. Gottlow J. Guided tissue regeneration using bioresorbable and non-resorbable devices: Initial healing and long-term results. J Periodontol. 1993;64:1157–1165.
7. Bunyaratavej P, Wang HL. Collagen membranes: A review. J Periodontol. 2001; 72:215–229.
8. Zitzmann NU, Naef R, Scharer P. Resorbable versus non-resorbable membranes in combination with Bio-Oss for guided bone regeneration. Int J Oral MaxillofacImplants. 1997;12:844–852.
9. Zubery Y, Nir E, Goldlust A. Ossification of a collagen membrane cross-linked by sugar: a human case series. J Periodontol. 2008;79(6):1101–1107.
10. Friedmann A, Gissel K, Soudan M, Kleber BM, Pitaru S, Dietrich T. Randomized controlled trial on lateral augmentation using two collagen membranes: morphometric results on mineralized tissue compound. J Clin Periodontol. 2011;38(7):677–685.
11. Neiva R, Pagni G, Duarte F, Park C, Yi E, Holman L, Giannobile W. Analysis of tissue neogenesis in extraction sockets treated with guided bone regeneration: clinical, histologic, and micro-CT results. Int J Periodontics Restorative Dent. 2011;31(5):457–469.
12. Klinger A, Asad R, Shapira L, Zubery Y. In vivo degradation of collagen barrier membranes exposed to the oral cavity. Clin Oral Implants Res. 2010;21(8):873–876.
13. Sterio TW, Katancik JA, Blanchard SB, Xenoudi P, Mealey BL. A prospective, multicenter study of bovine pericardium membrane with cancellous particulate allograft for localized alveolar ridge augmentation. Int J Periodontics Restorative Dent. 2013;33(4):499–507.
Thomas Sterio, DMD, MS, graduated from the University of Connecticut School of Dental Medicine and completed his residency training and certificate in periodontics and dental implants at the University of Texas Health Science Center in San Antonio. He completed a master’s degree in biomedical sciences and received special training and certification in advanced sedation techniques, including intravenous conscious sedation. Dr. Sterio is a diplomate of the American Board of Periodontology and was the recipient of the American Academy of Periodontology’s Dr. and Mrs. Gerald M. Kramer Scholar Award for Excellence, given to the nation’s top overall graduating resident. He is a guest lecturer for the Harvard University and Tufts University Department of Periodontics. Dr. Sterio enjoys spending time with his family and their chocolate Labradors, running, following the local sports teams, and reading.
Disclosure: The author received funding for this article from OraPharma, a division of Valeant Pharmaceuticals International Inc.